MPGPG02A1
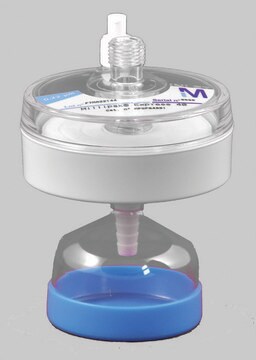
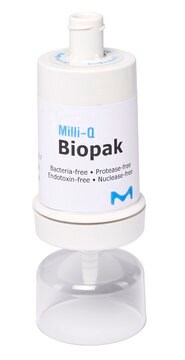
Millipak® Gold Filter
For particulates- and bacteria-free water at the point of dispense of the Milli-Q® 7 series water purification systems.
Synonym(s):
Millipak® Gold 0.22 μm Sterile Filter
About This Item
Recommended Products
material
polyethersulfone filter
Quality Level
sterility
sterile
feature
hydrophilic
packaging
pkg of 1 unit
manufacturer/tradename
Millipak®
outlet connection diam.
1/4 in.
pore size
0.22 μm pore size
fitting
outlet stepped hose barb (with bell)
compatibility
for use with Milli-Q® EQ 7000
for use with Milli-Q® EQ 7008
for use with Milli-Q® EQ 7016
for use with Milli-Q® IX 7003
for use with Milli-Q® IX 7005
for use with Milli-Q® IX 7010
for use with Milli-Q® IX 7015
for use with Milli-Q® IQ 7000
for use with Milli-Q® IQ 7003
for use with Milli-Q® IQ 7005
for use with Milli-Q® IQ 7010
for use with Milli-Q® IQ 7015
shipped in
ambient
Looking for similar products? Visit Product Comparison Guide
General description
The membrane in the Millipak® filter consists of conical pores that allow the water to filter at a higher flow rate with low differential pressure. The filter is heat-sealed on SAN (styrene-acrylonitrile) housing material. The housing material, along with the membrane and the production processes are carefully chosen to reduce organic and inorganic extractable release.
Millipak® Gold 0.22 μm sterile filter is suitable for use with Milli-Q® IQ 7000, Milli-Q® IQ 7003/05/10/15, Milli-Q® IX 7003/05/10/15 and Milli-Q® EQ 7000/08/16 water purification systems.
Application
Features and Benefits
- Each Millipak® Gold 0.22 μm filter is individually tested, hermetically sealed and delivered with a Certificate of Quality.
- Delivers bacteria-free water at <0.01 cfu/mL when installed and used in a laminar flow hood.
- Delivers particulate-free water (no particles with size >0.22 μm).
- Carefree maintenance: Easily installed and replaced.
- Redesigned bottom-tip for a secured installation of the protection bell.
- e-Sure tag for RFID connection with E-POD® or Q-POD® dispenser enables full traceability (data management) and automatic consumable status monitoring on the POD′s touchscreen interface.
Other Notes
- Organism Retention: Microorganisms
- Mode of Action: Filtering
- Application: General laboratory analysis
- Intended Use: Water purification
- Instructions for Use: This item provides water filtered through 0.22 μm sterilizing-grade membranes. Refer to the system equipment user guide section "Using the System" (available on USB key in the system box).
- Storage Statement: Store in a dry location
- Disposal Statement: Dispose of in accordance with applicable federal, state and local regulations.
Legal Information
also commonly purchased with this product
Storage Class
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
See how two water sources compare when preparing and performance testing microbiological culture media acc. to EN ISO 11133: centrally purified water vs. water from a Milli-Q® IX system were assessed. Tests included media specific for Listeria and Salmonella pathogens.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service