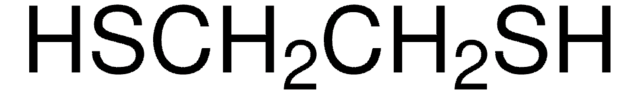8.00795
1,2-Ethanedithiol
for synthesis
Synonym(s):
1,2-Ethanedithiol
About This Item
Recommended Products
vapor pressure
6.4 hPa ( 20 °C)
Quality Level
assay
≥99.0% (GC)
form
liquid
potency
120 mg/kg LD50, oral (Rat)
197 mg/kg LD50, skin (Rabbit)
mp
-41 °C
transition temp
flash point 50 °C
density
1.12 g/cm3 at 20 °C
storage temp.
2-30°C
InChI
1S/C2H6S2/c3-1-2-4/h3-4H,1-2H2
InChI key
VYMPLPIFKRHAAC-UHFFFAOYSA-N
Application
- Aryl thiols via copper-catalyzed single-step reaction with aryl halides.
- 1,3-Dithiolanes via Lewis acid-catalyzed chemoselective condensation with carbonyl compounds.
- Polyalkylene sulfides via polycondensation with dibromides in the presence of NaH.
Analysis Note
Density (d 20 °C/ 4 °C): 1.123 - 1.126
Identity (IR): passes test
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
111.2 °F - closed cup
flash_point_c
44 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Related Content
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service