196878-M
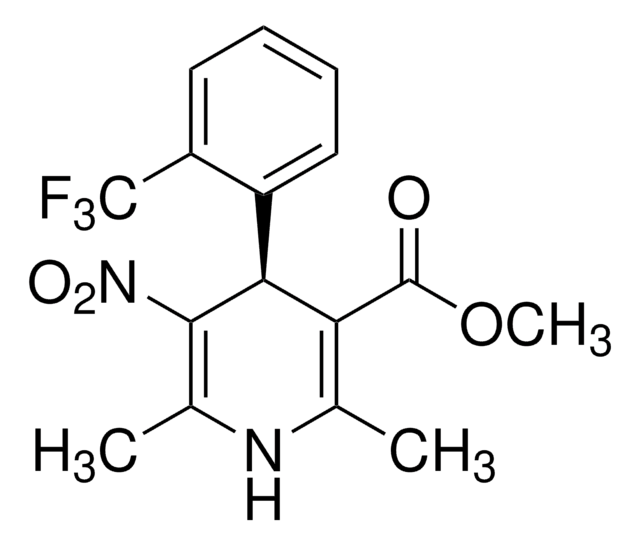
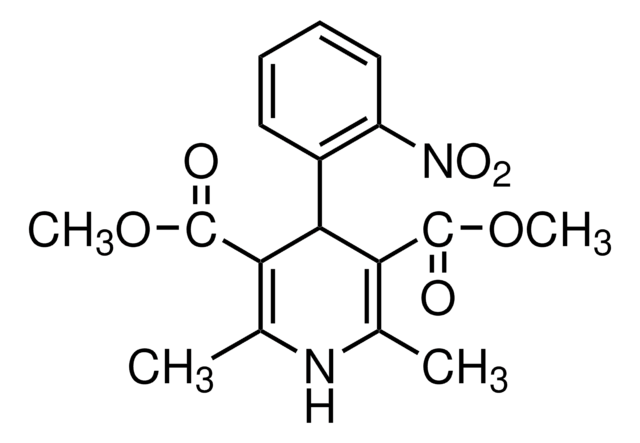
(±)-Bay K 8644
Synthetic dihydropyridine derivative that acts as an active Ca2+ slow channel agonist in neuroendocrine, muscle, thyroid and other cell types.
Synonym(s):
(±)-Bay K 8644, 1,4-Dihydro-2,6-dimethyl-5-nitro-4-[2ʹ-(trifluoromethyl)phenyl]-3-pyridinecarboxylic Acid Methyl Ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H15F3N2O4
Molecular Weight:
356.30
UNSPSC Code:
12352200
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
yellow
solubility
DMSO: 35 mg/mL
ethanol: 35 mg/mL
storage temp.
2-8°C
General description
A synthetic dihydropyridine derivative that is an active Ca2+ slow channel agonist in neuroendocrine, muscle, thyroid, and other cell types. Prolongs single channel open time without affecting the close time. An L-type Ca2+ channel agonist. Composed of two optical isomers. The (-)-enantiomer has strong vasoconstrictive, positive ionotropic, and Ca2+ agonistic properties, whereas the (+)-enantiomer has weak vasodilating, negative ionotropic, and Ca2+ antagonistic properties. The net effect of the racemic mix is that of the negative enantiomer. Promotes β-cell proliferation/regeneration.
Synthetic dihydropyridine derivative that acts as an active Ca2+ slow channel agonist in neuroendocrine, muscle, thyroid and other cell types. Prolongs single channel open time without affecting the close time. An L-type Ca2+ channel agonist. Composed of two optical isomers. The (-)-enantiomer has strong vasoconstrictive, positive inotropic, and Ca2+ agonistic properties, whereas the (+)-enantiomer has weakly vasodilating, negative inotropic, and Ca2+ antagonistic properties. The net effect of the racemic mix is that of the negative enantiomer. Promotes β-cell proliferation/regeneration.
Biochem/physiol Actions
Primary Target
L-type Ca2+ channel
L-type Ca2+ channel
Warning
Toxicity: Irritant (B)
Reconstitution
Following reconstitution, store in the refrigerator (4°C). Stock solutions are stable for up to 20 days at 4°C.
Other Notes
Wang, W., et al. 2009. Proc. Natl. Acad. Sci. USA106, 1427.
Weigl, L.G., et al. 2000. J. Physiol.525 (pt. 2), 461.
Vannier, C., et al. 1995. Am. J. Physiol. 268, L201.
Bechem, M., and Hoffmann, H. 1993. Pflugers Arch. 424, 343.
Triggle, D.J., and Rompe, D. 1989. Trends Pharmacol. Sci. 10, 507.
Takasu, N., et al. 1987. Biochem. Biophys. Res. Commun.143, 1107.
Tagliatela, M., et al. 1986. Brain Res.381, 356.
Franckowiak, G., et al. 1985. Eur. J. Pharmacol.114, 223.
Weigl, L.G., et al. 2000. J. Physiol.525 (pt. 2), 461.
Vannier, C., et al. 1995. Am. J. Physiol. 268, L201.
Bechem, M., and Hoffmann, H. 1993. Pflugers Arch. 424, 343.
Triggle, D.J., and Rompe, D. 1989. Trends Pharmacol. Sci. 10, 507.
Takasu, N., et al. 1987. Biochem. Biophys. Res. Commun.143, 1107.
Tagliatela, M., et al. 1986. Brain Res.381, 356.
Franckowiak, G., et al. 1985. Eur. J. Pharmacol.114, 223.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service