A78608
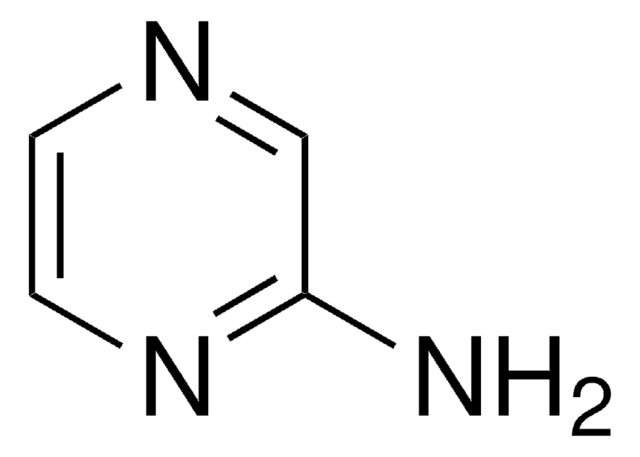
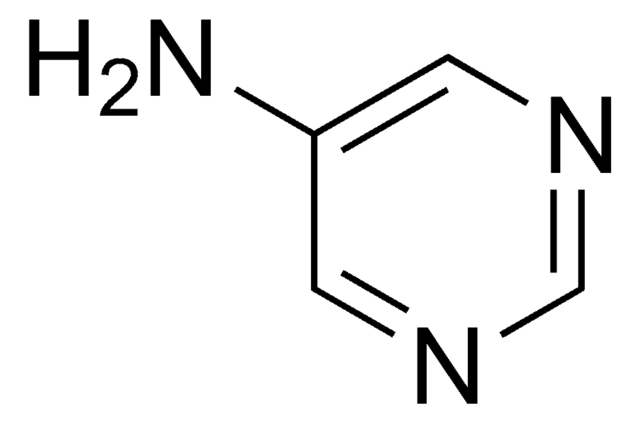
2-Aminopyrimidine
97%
Synonym(s):
2-Pyrimidinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein/REAXYS Number:
107014
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
powder
mp
122-126 °C (lit.)
SMILES string
Nc1ncccn1
InChI
1S/C4H5N3/c5-4-6-2-1-3-7-4/h1-3H,(H2,5,6,7)
InChI key
LJXQPZWIHJMPQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S A Abdel-Latif et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(3-4), 950-957 (2006-11-07)
The formation constants of some transition metal ions Cr(III), Mn(II), Fe(III), Ni(II) and Cu(II) binary complexes containing Schiff bases resulting from condensation of salicylaldehyde with aniline (I), 2-aminopyridine (II), 4-aminopyridine (III) and 2-aminopyrimidine (IV) were determined pH-metrically in ethanolic medium
Rogier A Smits et al.
Drug discovery today, 14(15-16), 745-753 (2009-05-30)
The search for new and potent histamine H4 receptor ligands is leading to a steadily increasing number of scientific publications and patent applications. Several interesting and structurally diverse compounds have been found, but fierce IP competition for a preferred 2-aminopyrimidine
Namrata Anand et al.
Bioorganic & medicinal chemistry, 20(17), 5150-5163 (2012-08-03)
A synthetic strategy to access small libraries of triazolylmethoxy chalcones 4{1-20}, triazolylmethoxy flavanones 5{1-10} and triazolylmethoxy aminopyrimidines 6{1-17} from a common substrate 4-propargyloxy-2-hydroxy acetophenone using a set of different reactions has been developed. The chalcones and flavanones were screened against
Jinho Lee et al.
Bioorganic & medicinal chemistry letters, 21(14), 4203-4205 (2011-06-21)
A series of new 2-(2-aminopyrimidin-4-yl)phenol derivatives were synthesized as potential antitumor compounds. Substitution with pyrrolidine-3,4-diol at the 4-position of phenol provided potent inhibitory activity against CDK1 and CDK2. X-ray crystal structural studies were performed to account for the effect of
Manishkumar D Joshi et al.
Journal of chromatography. A, 1308, 161-165 (2013-08-21)
A reversed-phase high performance liquid chromatography (HPLC) method is described for the determination of boronic acids that are commonly present as impurities in pinacolboronate ester reagents. Boronic acids and their pinacolboronate esters are key reagents in the Suzuki-Miyaura coupling reaction.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service