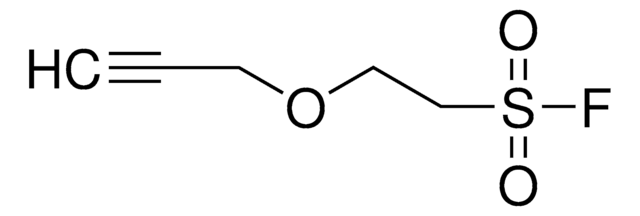
914231
4-(2-(Hex-5-ynamido)ethyl)benzenesulfonyl fluoride
≥95%
Synonym(s):
DAS1, SFABP, Sulfonyl fluoride activity-based probe
About This Item
Recommended Products
Quality Level
assay
≥95%
form
powder
storage temp.
−20°C
SMILES string
F[S](=O)(=O)c1ccc(cc1)CCNC(=O)CCCC#C
InChI
1S/C14H16FNO3S/c1-2-3-4-5-14(17)16-11-10-12-6-8-13(9-7-12)20(15,18)19/h1,6-9H,3-5,10-11H2,(H,16,17)
InChI key
HWSJGTCAHFQVSV-UHFFFAOYSA-N
Application
Other Notes
Sulfonyl fluorides as privileged warheads in chemical biology
Chemical Proteomics with Sulfonyl Fluoride Probes Reveals Selective Labeling of Functional Tyrosines in Glutathione Transferase
Sulfonyl Fluoride Analogues as Activity-Based Probes for Serine Proteases
Europium-Labeled Activity-Based Probe through Click Chemistry: Absolute Serine Protease Quantification Using 153Eu Isotope Dilution ICP/MS
Related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 914231-50MG | 4061842093311 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service