All Photos(2)
About This Item
Empirical Formula (Hill Notation):
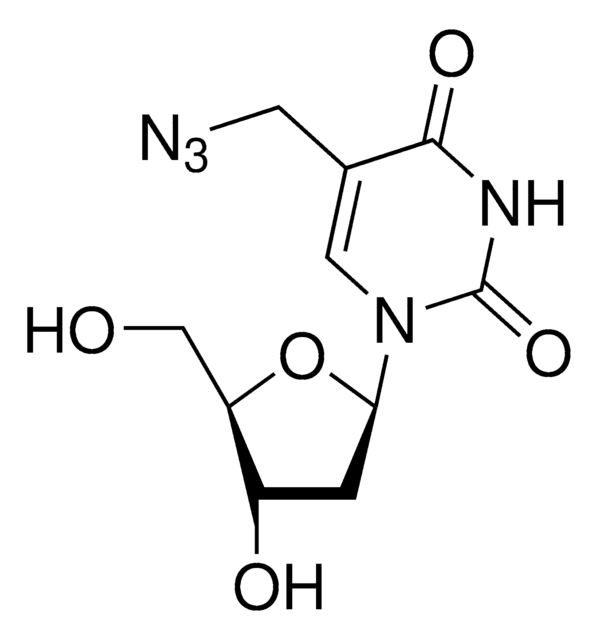
C11H12N2O5
CAS Number:
Molecular Weight:
252.22
MDL number:
UNSPSC Code:
12352208
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
powder
mp
206 °C
storage temp.
−20°C
SMILES string
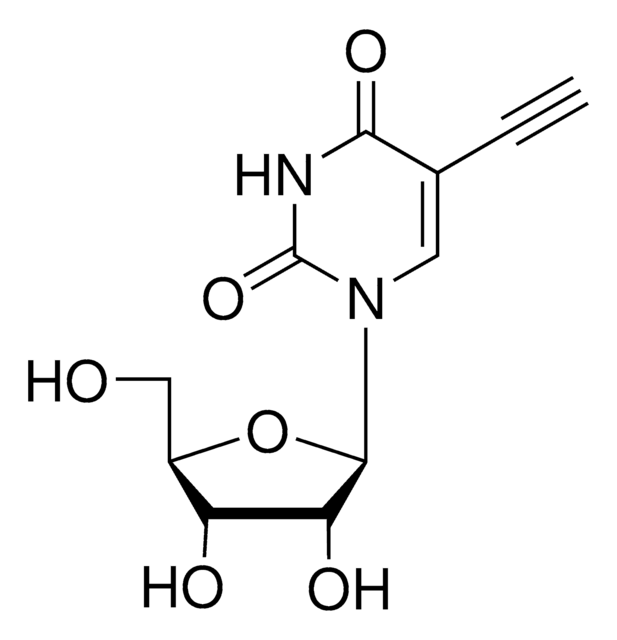
OC[C@@H]1[C@@H](O)C[C@H](N2C(NC(C(C#C)=C2)=O)=O)O1
InChI
1S/C11H12N2O5/c1-2-6-4-13(11(17)12-10(6)16)9-3-7(15)8(5-14)18-9/h1,4,7-9,14-15H,3,5H2,(H,12,16,17)/t7-,8+,9+/m0/s1
InChI key
CDEURGJCGCHYFH-DJLDLDEBSA-N
Related Categories
General description
5-Ethynyl-2′-deoxyuridine (EdU) is a thymidine analogue that can be incorporated into cellular DNA for cell proliferation studies. The incorporated nucleoside analogue can be detected by a copper-catalyzed click reaction with a fluorescent azide.
Application
5-Ethynyl-2′-deoxyuridine (EdU) is used for:
For a listing of the Baseclick kits see: Baseclick kits
- Labeling newly synthesized DNA during replication
- Cell proliferating assay studies
- Cell cycle analysis
For a listing of the Baseclick kits see: Baseclick kits
signalword
Danger
hcodes
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, susceptibility to enzymatic phosphorylation, cytotoxicity and in vitro antiviral activity of lipophilic pyrimidine nucleoside/carborane conjugates.
Bialek-Pietras M, et al.
Journal of Organometallic Chemistry, 865, 166-172 (2018)
5-Ethynyl-2?-deoxycytidine as a new agent for DNA labeling: detection of proliferating cells
D Qu, et.al.
Analytical Biochemistry, 417, 112-121 (2011)
Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA.
Gierlich J, et al.
Organic Letters, 8(17), 3639-3642 (2006)
Site-directed spin-labeling of nucleic acids by click chemistry: detection of abasic sites in duplex DNA by EPR spectroscopy.
Jakobsen A, et al.
Journal of the American Chemical Society, 132(30), 10424-10428 (2010)
Timothy J Mead et al.
Methods in molecular biology (Clifton, N.J.), 1130, 233-243 (2014-02-01)
Assessing cell proliferation in situ is an important phenotyping component of skeletal tissues from development to adult stages and disease. Various methods exist including immunostaining for proteins and protein modifications associated with specific steps of the cell cycle, but the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 900584-500MG | 4061833250372 |
| 900584-50MG | 4061833250389 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service