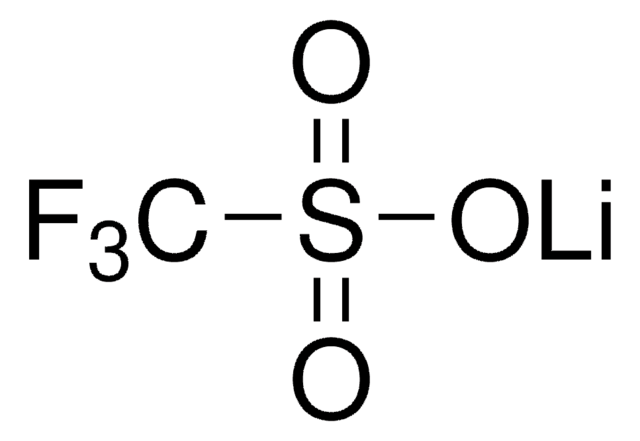
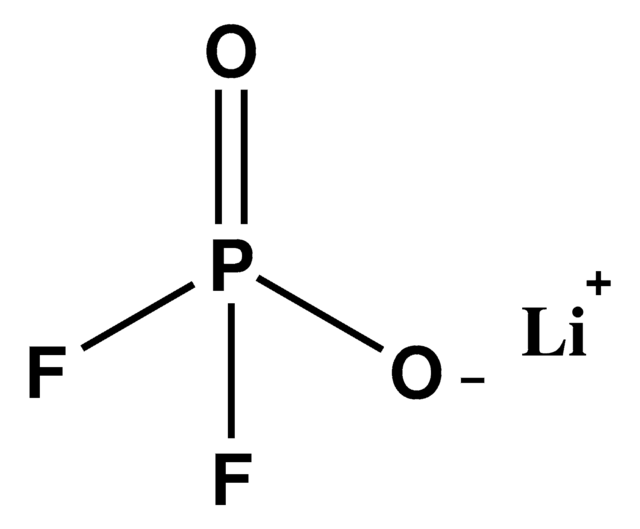
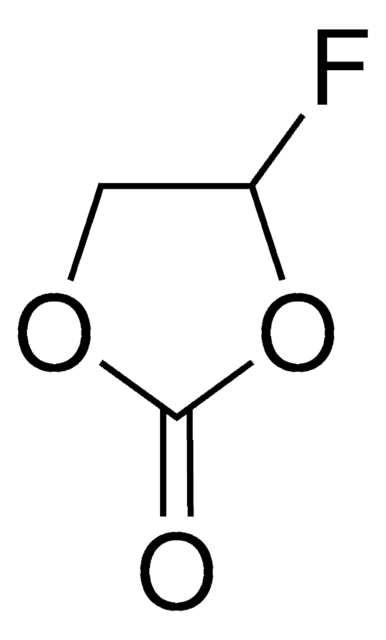
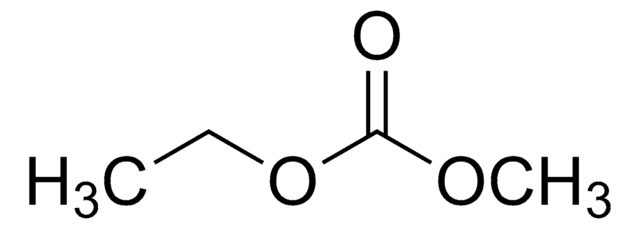
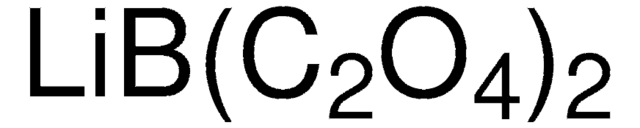774138
Lithium difluoro(oxalato)borate
Synonym(s):
LIDFOB, LIF2OB, LIFOB, LIODFB, Lithium difluoro(ethanedioato)borate, Lithium oxalatodigluoroborate
About This Item
Recommended Products
form
powder
Quality Level
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
265-271 °C
application(s)
battery manufacturing
greener alternative category
, Enabling
SMILES string
F[B-]1(OC(C(O1)=O)=O)F.[Li+]
InChI
1S/C2BF2O4.Li/c4-3(5)8-1(6)2(7)9-3;/q-1;+1
InChI key
MEDDCIKGDMDORY-UHFFFAOYSA-N
General description
Application
Features and Benefits
✔ Increases battery life
✔ Stabilizes SEI layer
✔ Suitable for fast charging and low temperatures
Related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service