757144
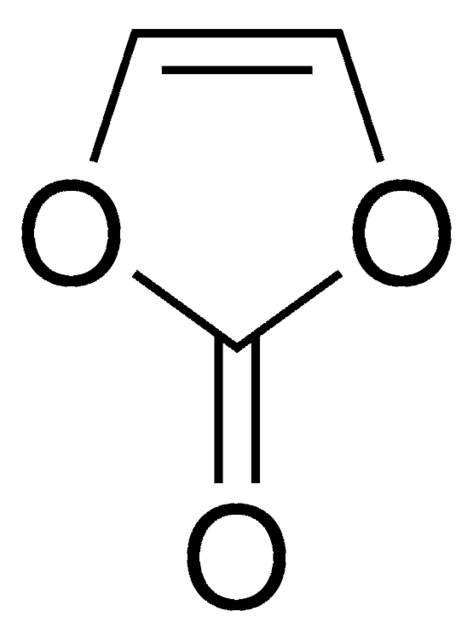
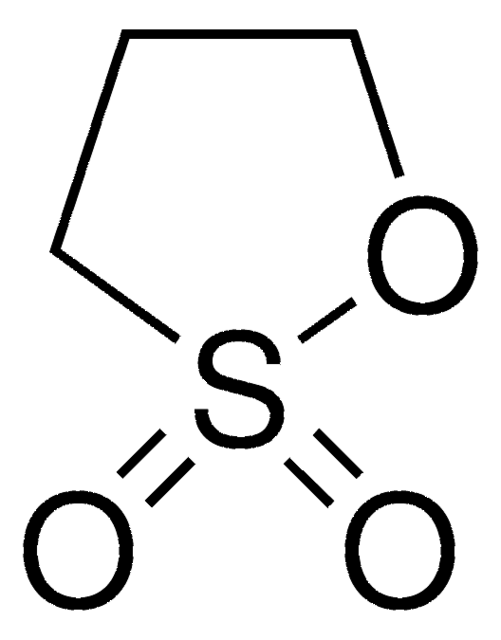
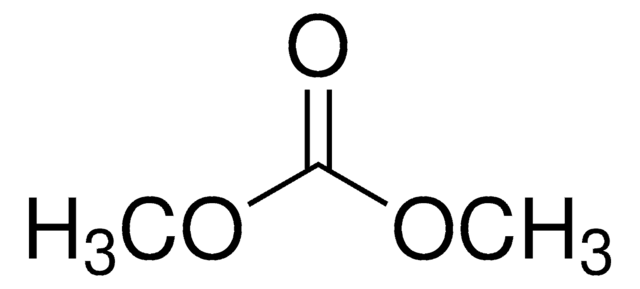
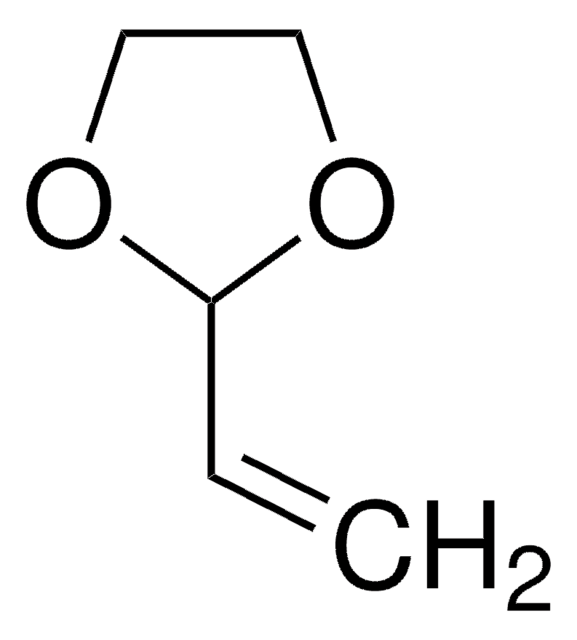
Vinylene carbonate
contains 80 ppm BHT as stabilizer, 99%
Synonym(s):
1,3-Dioxol-2-one, VC
About This Item
Recommended Products
assay
99%
form
liquid
contains
80 ppm BHT as stabilizer
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.421 (lit.)
n20/D 1.422
bp
162 °C (lit.)
mp
19-22 °C (lit.)
density
1.355 g/mL at 25 °C (lit.)
1.355 g/mL at 25 °C
application(s)
battery manufacturing
greener alternative category
, Enabling
storage temp.
2-8°C
SMILES string
O=C1OC=CO1
InChI
1S/C3H2O3/c4-3-5-1-2-6-3/h1-2H
InChI key
VAYTZRYEBVHVLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
target_organs
Liver,Stomach
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
181.9 °F - closed cup
flash_point_c
83.3 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Li-ion batteries are currently the focus of numerous research efforts with applications designed to reduce carbon-based emissions and improve energy storage capabilities.
Discover more about advancements being made to improve energy density of lithium ion battery materials.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service