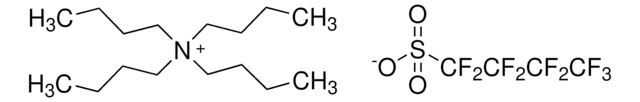
727989
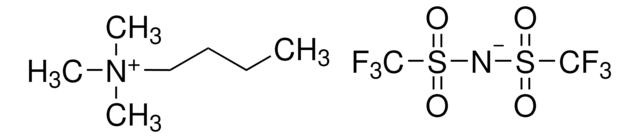
Ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18F6N2O4S2
CAS Number:
Molecular Weight:
396.37
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
impurities
≤1.0% water
functional group
amine
fluoro
SMILES string
CCC[N+](C)(C)CC.FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F
InChI
1S/C7H18N.C2F6NO4S2/c1-5-7-8(3,4)6-2;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h5-7H2,1-4H3;/q+1;-1
InChI key
FKXJTTMLNYZAOH-UHFFFAOYSA-N
Related Categories
General description
Ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide is an ionic liquid with high dielectrical permittivity, ionic conductivity and low viscosity.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermochemical properties of 1-butyl-3-methylimidazolium nitrate
Strechan, A. A., et al.
Electrochimica Acta, 474.1, 25-31 (2008)
Electrical double layer capacitors with sucrose derived carbon electrodes in ionic liquid electrolytes.
Wei L and Yushin G.
Journal of Power Sources, 196(8), 4072-4079 (2011)
Parametrization of 1-butyl-3-methylimidazolium hexafluorophosphate/nitrate ionic liquid for the GROMOS force field
Micaelo, Nuno M., Antonio M. Baptista, and Claudio M. Soares
Analytical Chemistry, 110.29, 14444-14451 (2006)
Density and viscosity of 1-butyl-3-methylimidazolium nitrate with ethanol, 1-propanol, or 1-butanol at several temperatures
Mokhtarani, Babak, et al.
Analytical Chemistry, 41.12, 1432-1438 (2009)
Abderrahman Atifi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(53), 13076-13086 (2017-07-26)
The solvent environment around iron porphyrin complexes was examined using mixed molecular/RTIL (room temperature ionic liquid) solutions. The formation of nanodomains in these solutions provides different solvation environments for substrates that could have significant impact on their chemical reactivity. Iron
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
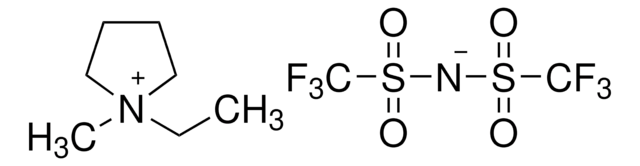
![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)