721433
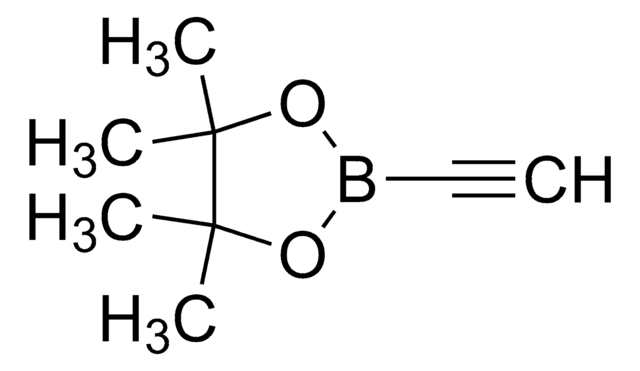
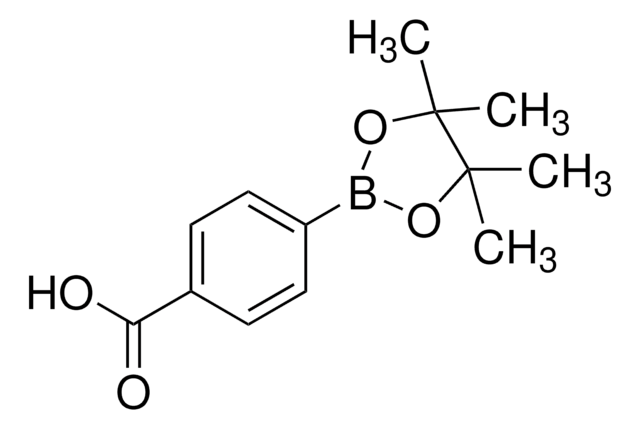
4-Ethynylphenylboronic acid pinacol ester
95%
Synonym(s):
2-(4-Ethynylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H17BO2
CAS Number:
Molecular Weight:
228.09
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
form
solid
mp
66-70 °C
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(cc2)C#C
InChI
1S/C14H17BO2/c1-6-11-7-9-12(10-8-11)15-16-13(2,3)14(4,5)17-15/h1,7-10H,2-5H3
InChI key
LOVNTFMVZVIASV-UHFFFAOYSA-N
Application
4-Ethynylphenylboronic acid pinacol ester can be used:
- For the functionalization of platinum nanoparticles to leverage their photoluminescence properties.
- As an intermediate in the synthesis of covalent heterodyads of chlorophyll derivatives applicable as supramolecular light-harvesting systems.
- As an intermediate in the preparation of F-18 radiolabeled galectin-3 inhibitors, which are used as surrogate positron emission tomography (PET) tracers of TD139 and GB1107.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service