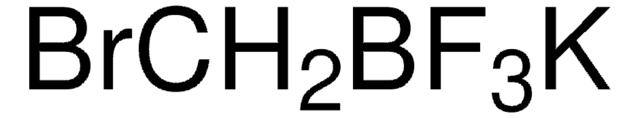About This Item
Recommended Products
form
solid
Quality Level
mp
245-258 °C (dec.)
functional group
iodo
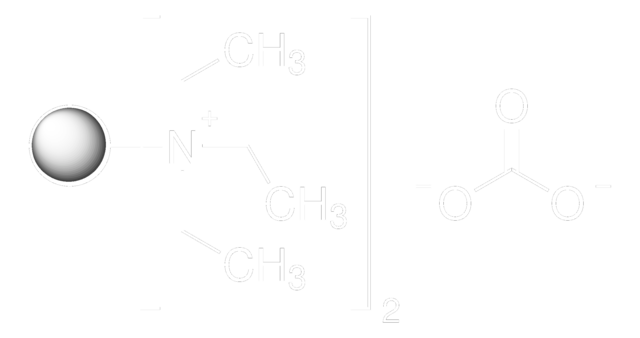
SMILES string
[K+].C[B-](C)(C)CI
InChI
1S/C4H11BI.K/c1-5(2,3)4-6;/h4H2,1-3H3;/q-1;+1
InChI key
YPGDEVDDTXBNRH-UHFFFAOYSA-N
Related Categories
Application
Organotrifluoroboronates as versatile and stable boronic acid surrogates.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service