70956
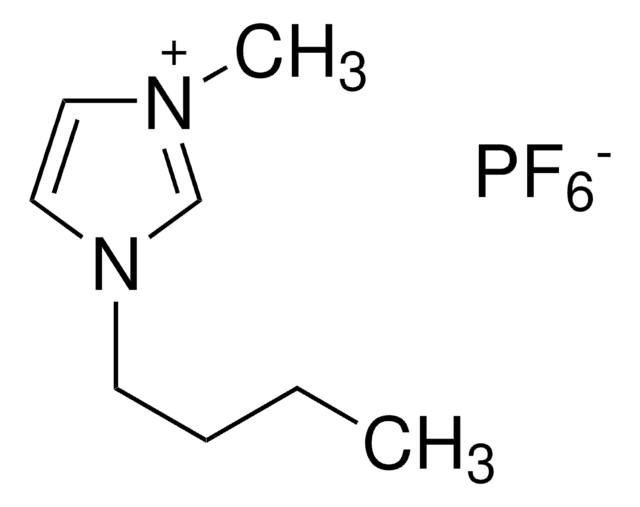
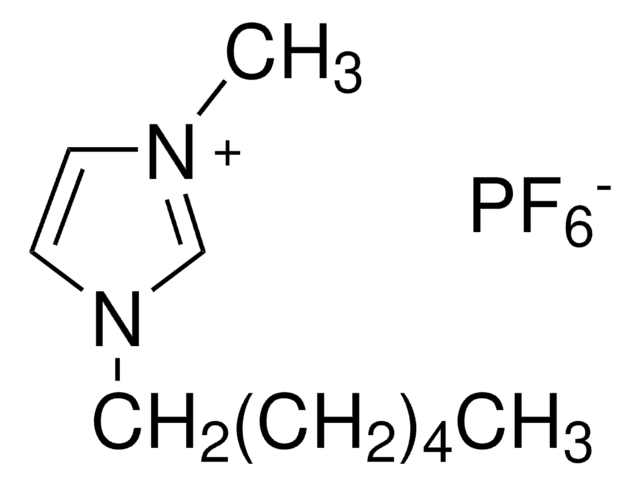
1-Butyl-3-methylimidazolium hexafluorophosphate
≥97.0% (HPLC)
Synonym(s):
BMIMPF6
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15F6N2P
CAS Number:
Molecular Weight:
284.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (HPLC)
form
liquid
refractive index
n20/D 1.411 (lit.)
density
1.38 g/mL at 20 °C (lit.)
SMILES string
F[P-](F)(F)(F)(F)F.CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.F6P/c1-3-4-5-10-7-6-9(2)8-10;1-7(2,3,4,5)6/h6-8H,3-5H2,1-2H3;/q+1;-1
InChI key
IXQYBUDWDLYNMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
BMIMPF6 is used as an efficient reaction medium for various synthetic and catalytic transformations.
1-Butyl-3-methylimidazolium hexafluorophosphate is an imidazolium-based, hydrophobic, room temperature ionic liquid (RTIL). It can be prepared by reacting 1-methylimidazole with chlorobutane. Gaseous hydrofluorocarbons (HFCs) such as fluoromethane, fluoroethane and 1,1,2,2-tetrafluoroethane are soluble in BMIMPF6.
1-Butyl-3-methylimidazolium hexafluorophosphate is an imidazolium-based, hydrophobic, room temperature ionic liquid (RTIL). It can be prepared by reacting 1-methylimidazole with chlorobutane. Gaseous hydrofluorocarbons (HFCs) such as fluoromethane, fluoroethane and 1,1,2,2-tetrafluoroethane are soluble in BMIMPF6.
Application
1-Butyl-3-methylimidazolium hexafluorophosphate is an ionic liquid employed in many environmentally friendly reactions.
It can also be used as a medium for reactions such as:
It can also be used as a medium for reactions such as:
- Ring-closing metathesis of diene and enyne substrates in the presence of a novel recyclable ruthenium carbene complex.
- Nickel(II)acetylacetonate catalyzed oxidation of aromatic aldehydes to the corresponding acids using dioxygen as the oxidant.
- Lipase-catalyzed enantioselective acylation of allylic alcohols.
- Allylation of aldehydes using tetraallylstannane to yield homoallylic alcohols.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gaseous absorption of fluoromethane, fluoroethane, and 1, 1, 2, 2-tetrafluoroethane in 1-butyl-3-methylimidazolium hexafluorophosphate.
Shiflett MB and Yokozeki A.
Industrial & Engineering Chemistry Research, 45(18), 6375-6382 (2006)
Gulcin Bolat et al.
Sensors (Basel, Switzerland), 18(3) (2018-03-08)
Malathion (MLT) is an organophosphorous type pesticide and having seriously high toxicity and electrochemical platforms for rapid, simple, inexpensive and sensitive determination of pesticides is still a special concern. This paper describes a simple preparation of a composite film consisting
Oxidation of aromatic aldehydes in the ionic liquid [bmim] PF6.
Howarth J.
Tetrahedron Letters, 41(34), 6627-6629 (2000)
Ionic liquids: a convenient solvent for environmentally friendly allylation reactions with tetraallylstannane.
Gordon C.
Chemical Communications (Cambridge, England), (15), 1431-1432 (1999)
Olefin Metathesis in the Ionic Liquid 1?Butyl?3?methylimidazolium Hexafluorophosphate Using a Recyclable Ru Catalyst: Remarkable Effect of a Designer Ionic Tag.
Yao Q and Zhang Y
Angewandte Chemie (International Edition in English), 42(29), 3395-3398 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service