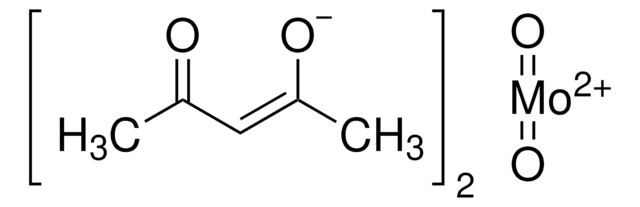
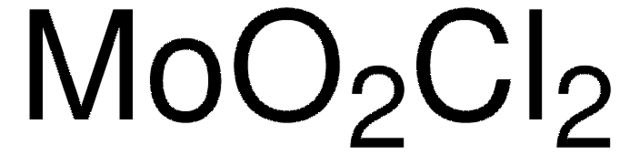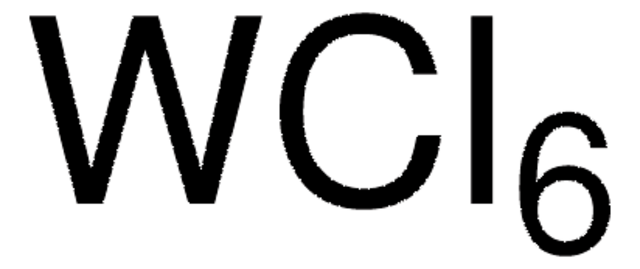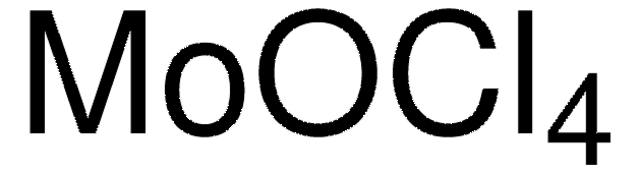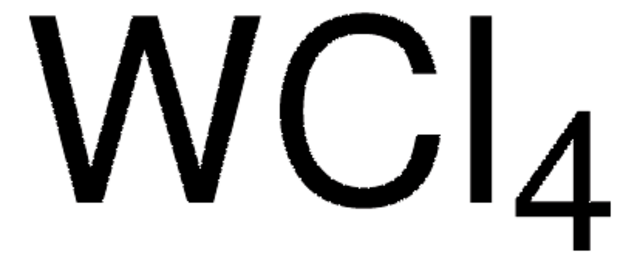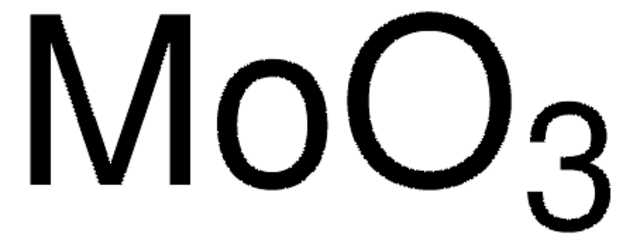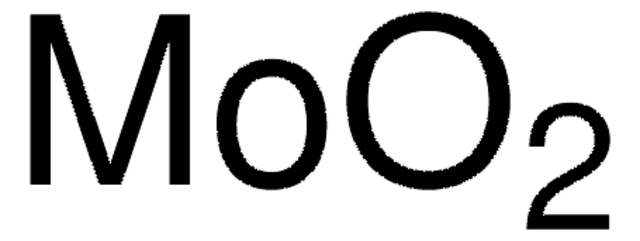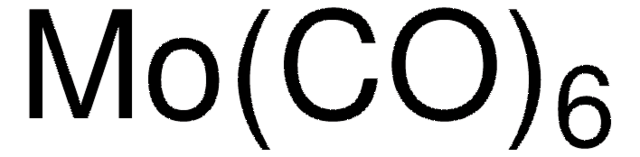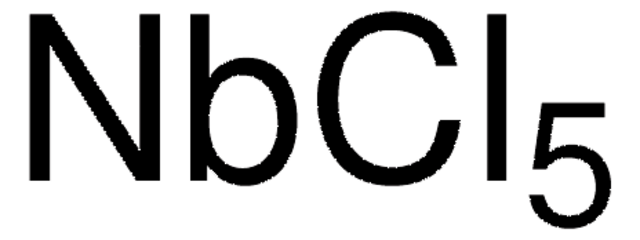642452
Molybdenum(V) chloride
anhydrous, powder, 99.99% trace metals basis (excluding W)
Synonym(s):
Molybdenum pentachloride, Molybdenum(5+) chloride
About This Item
Recommended Products
grade
anhydrous
Quality Level
vapor pressure
1.75 mmHg ( 25 °C)
131 mmHg ( 250 °C)
assay
99.99% trace metals basis (excluding W)
form
powder
impurities
≤150.0 ppm Trace Metal Analysis
bp
268 °C (lit.)
mp
194 °C (lit.)
density
2.928 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
Cl[Mo](Cl)(Cl)(Cl)Cl
InChI
1S/5ClH.Mo/h5*1H;/q;;;;;+5/p-5
InChI key
GICWIDZXWJGTCI-UHFFFAOYSA-I
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a catalyst for amidation of secondary benzyl alcohols.
- As a precursor to fabricate MoS2 thin films by atomic layer deposition method.
- As a primary catalyst for coordination polymerization of butadiene.
- To fabricate superior anode materials for Na-ion and Li-ion batteries.
- As a dual-function redox mediator for Li–O2 batteries to overcome thehigh polarization and low energy density issues.
accessory
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
We presents an article about Copper(I)-mediated Living Radical Polymerization in the Presence of Pyridylmethanimine Ligands, and the emergence of living radical polymerization mediated by transition metal catalysts in 1995, which was a seminal piece of work in the field of synthetic polymer chemistry.
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Related Content
We offer a complete line of the highest purity inorganic salts and materials for the micro and nanoelectronics market.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 642452-2G | 4061832729268 |
| 642452-10G | 4061833599785 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service