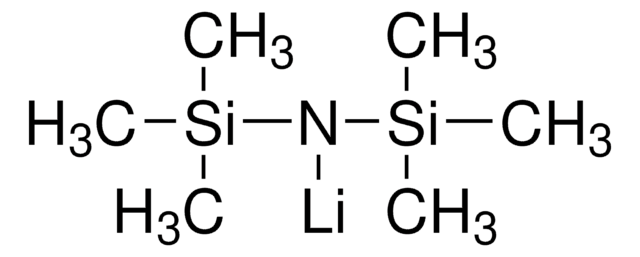577928
Lithium bis(trimethylsilyl)amide solution
1 M in toluene
Synonym(s):
LiHMDS, Hexamethyldisilazane lithium salt
About This Item
Recommended Products
Quality Level
concentration
1 M in toluene
density
0.860 g/mL at 25 °C
SMILES string
[Li]N([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C6H18NSi2.Li/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
InChI key
YNESATAKKCNGOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the deprotonation and nucleophilic difluoromethylation reactions.
- To synthesize isoquinoline derivatives by the addition of N-iodosuccinimide (NIS) to the α-benzyl tosylmethyl isocyanides.
- To prepare arylboronic acid pinacol esters by the reaction of aryl fluorides with bis(pinacolato)diboron via palladium-catalyzed cross-coupling reaction.
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Self-heat. 1 - Skin Corr. 1B - STOT RE 2 - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
48.0 °F - closed cup
flash_point_c
8.9 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service