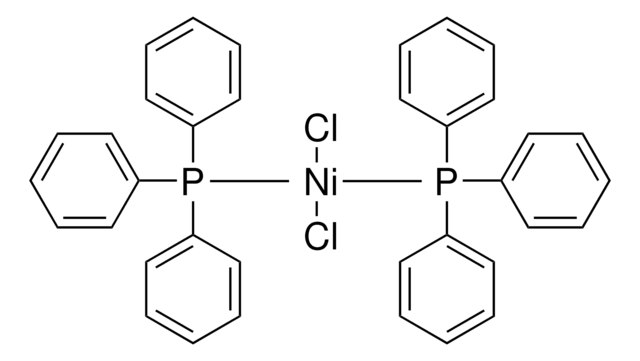
567671
Dichlorobis(trimethylphosphine)nickel(II)
97%
Synonym(s):
Bis(trimethylphosphine)nickel dichloride
About This Item
Recommended Products
assay
97%
form
solid
reaction suitability
core: nickel
reagent type: catalyst
mp
194-199 °C (lit.)
λmax
244 nm
SMILES string
Cl[Ni]Cl.CP(C)C.CP(C)C
InChI
1S/2C3H9P.2ClH.Ni/c2*1-4(2)3;;;/h2*1-3H3;2*1H;/q;;;;+2/p-2
InChI key
KYHNNWWCXIOTKC-UHFFFAOYSA-L
Application
- Wenkert arylation of thiophene with aryl Grignard reagents
- Regioselective [2+2+2] cycloaddition of carboryne with alkynes to give benzocarborane compounds
- Kumada-Corriu cross coupling of Grignard reagents
- Borylation of aryl chlorides
- Reductive aldol cyclization-lactonization
- Arylcyanation of alkynes
- Alkynylation of benzonitriles via C-C bond activation
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nickel transition metal and its complexes can be used as a catalyst in many synthetic transformations, like oxidative addition, C-H activation, reductive elimination, oxidative cyclization, oligomerization, and in cross-coupling reactions.
Related Content
Atto dyes are a series of fluorescent dyes that meet the critical needs of modern fluorescent technologies
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)
![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)