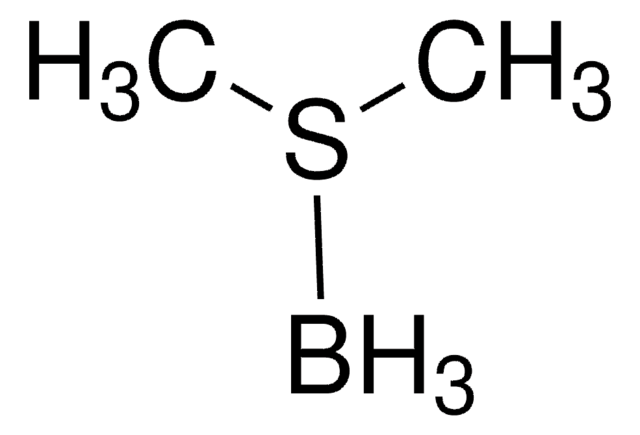
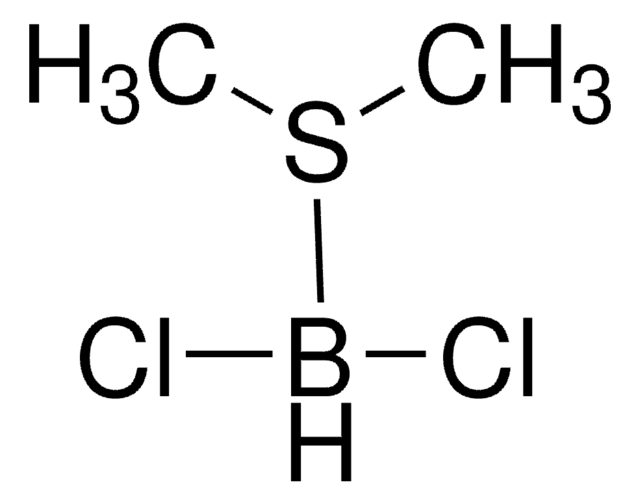
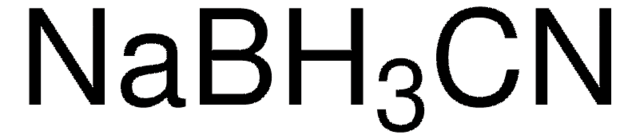555959
Dichloroborane dioxane complex solution
3 M in methylene chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C4H8O2 · BHCl2
CAS Number:
Molecular Weight:
170.83
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reagent type: reductant
Quality Level
concentration
3 M in methylene chloride
density
1.321 g/mL at 25 °C
functional group
ether
storage temp.
2-8°C
SMILES string
Cl[BH-](Cl)[O+]1CCOCC1
InChI
1S/C4H9BCl2O2/c6-5(7)9-3-1-8-2-4-9/h5H,1-4H2
InChI key
RCKKTUSYECYDLY-UHFFFAOYSA-N
Related Categories
Application
Reactant for:
- Hydroboration reactions
- Preparation of alkenyl- and alkylboronic acids
Can readily substitute for common hydroborating reagents such as BH3 · THF and BMS.
Legal Information
U.S. Pat. No. 6,008,414
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
221.0 °F - closed cup
flash_point_c
105 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J V Kanth et al.
The Journal of organic chemistry, 66(16), 5359-5365 (2001-08-04)
Several less volatile oxygen-containing Lewis bases, such as tert-butyl methyl ether, dioxane, anisole, ethyl acetate, beta-chloroethyl ether, and monoglyme, were examined as prospective mono- and dichloroborane carriers. Dioxane, ethyl acetate, and beta-chloroethyl ether form relatively stable boron trichloride adducts, but
Kanth, J. V. B.; Brown, H. C.
Organic Letters, 1, 315-315 (1999)
Josyula, K. V. B. et al
Tetrahedron Letters, 44, 7789-7789 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service