All Photos(2)
About This Item
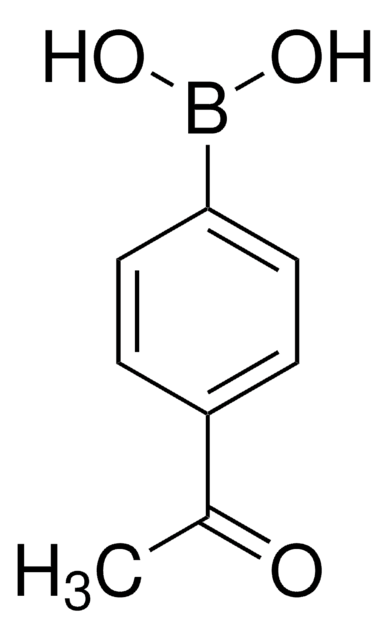
Linear Formula:
CH3COC6H4B(OH)2
CAS Number:
Molecular Weight:
163.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥95%
impurities
<10% 3-acetylphenylboronic anhydride
mp
204-208 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cccc(c1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-3-2-4-8(5-7)9(11)12/h2-5,11-12H,1H3
InChI key
SJGGDZCTGBKBCK-UHFFFAOYSA-N
Related Categories
Application
3-Acetylphenylboronic acid can be used as a substrate:
- In the synthesis of symmetric biaryls via oxidative dimerization using a palladium catalyst and water as a solvent.
- In the synthesis of aryl fluorides through electrophilic fluorination reaction using acetyl hypofluorite.
- In the coupling reactions of organoboranes with olefins using molecular oxygen and palladium catalyst.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fluorination of aryl boronic acids using acetyl hypofluorite made directly from diluted fluorine
Vints I, et al.
The Journal of Organic Chemistry, 78(23), 11794-11797 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)