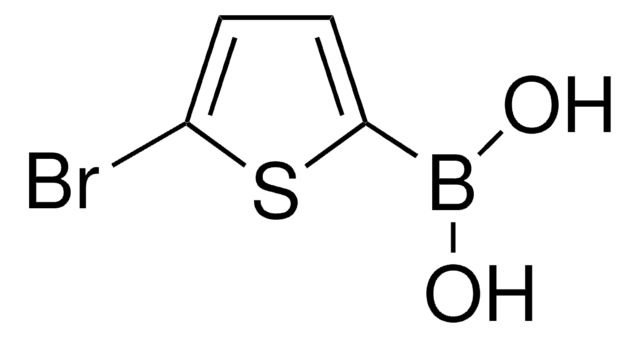
436836
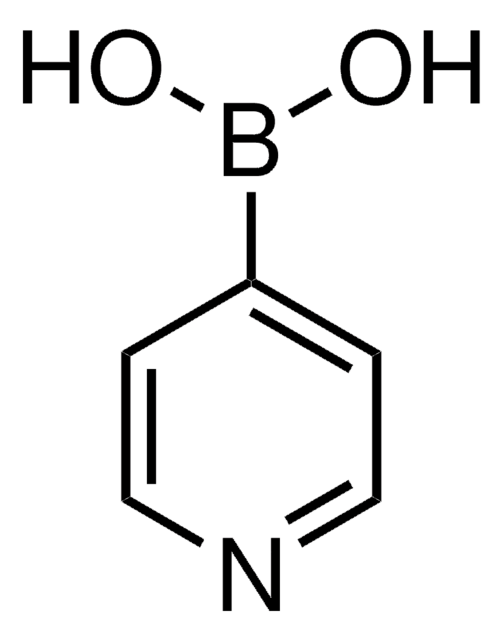
2-Thienylboronic acid
≥95.0%
Synonym(s):
2-Thienylboric acid, 2-Thienylboronic acid, Thien-5-ylboronic acid, Thiophene-2-boronic acid
About This Item
Recommended Products
Quality Level
assay
≥95.0%
form
solid
mp
138-140 °C (lit.)
storage temp.
2-8°C
SMILES string
OB(O)c1cccs1
InChI
1S/C4H5BO2S/c6-5(7)4-2-1-3-8-4/h1-3,6-7H
InChI key
ARYHTUPFQTUBBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Palladium-catalyzed Suzuki-Miyaura cross-couplings
- Alkylation, boration, coupling reaction, Suzuki coupling, and halogenation of fluorenyl bromide
- Chain-growth catalyst transfer polycondensation of conjugated alternating copolymer
- Ferric perchlorate-promoted reaction of fullerene to give fullerenyl boronic esters
- Ligand-free Suzuki, Sonogashira, and Heck cross-coupling reactions
- Copper-catalyzed nitration reactions
- Geometry relaxation-induced Large Stokes shift in red-emitting borondipyrromethenes (BODIPY) and applications in fluorescent thiol probes
Reagent used in Preparation of
- Photophysical properties of oxygen-containing polycyclic aromatic triptycenes
- Donor unit for donor-acceptor-type polymers via N-alkylation, Suzuki coupling, and bromination
- Aminopyridine-based inhibitors of mitotic kinase Nek2 with potential antipoliferative effects in cancer tumors
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)