All Photos(1)
About This Item
Empirical Formula (Hill Notation):
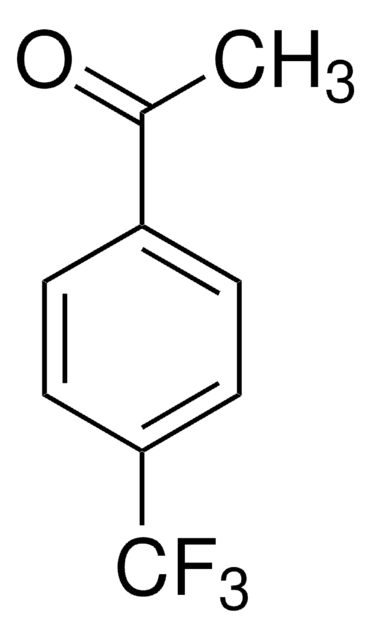
C6H4F3NO
CAS Number:
Molecular Weight:
163.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
mp
48-50 °C (lit.)
functional group
fluoro
ketone
SMILES string
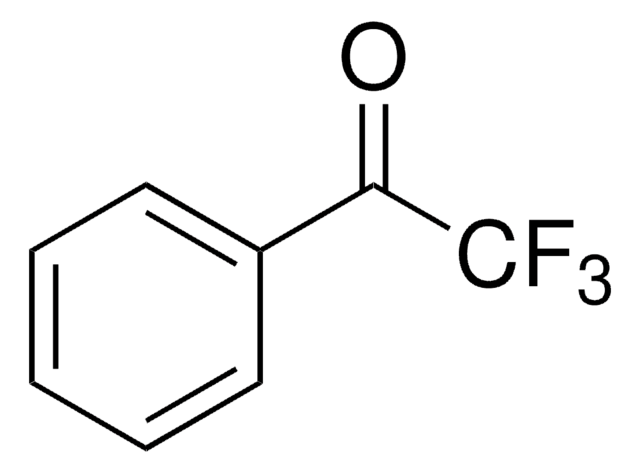
FC(F)(F)C(=O)c1ccc[nH]1
InChI
1S/C6H4F3NO/c7-6(8,9)5(11)4-2-1-3-10-4/h1-3,10H
InChI key
UMVVPYXSJKIFST-UHFFFAOYSA-N
Related Categories
General description
2-(Trifluoroacetyl)pyrrole, an α,α,α-trifluoromethyl ketone is a 2-substituted pyrrole derivative. It has been synthesized by reacting pyrrole with trifluoroacetic anhydride in benzene at 0°C. Its enantioselective hydrogenation to form corresponding alcohol using 5wt.% Pt/Al2O3 catalyst chirally modified by new synthetic chiral amines has been investigated.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chemo and enantioselective hydrogenation of fluorinated ketones on platinum modified with (R)-1-(1-naphthyl) ethylamine derivatives.
Diezi S, et al.
J. Mol. Catal. A: Chem., 239(1), 49-56 (2005)
Preparation of Indazoles and Quinazolines by Catalytic Dehydrogenation.
Burnett JrJ and Ainsworth C.
The Journal of Organic Chemistry, 23(9), 1382-1383 (1958)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service