107840
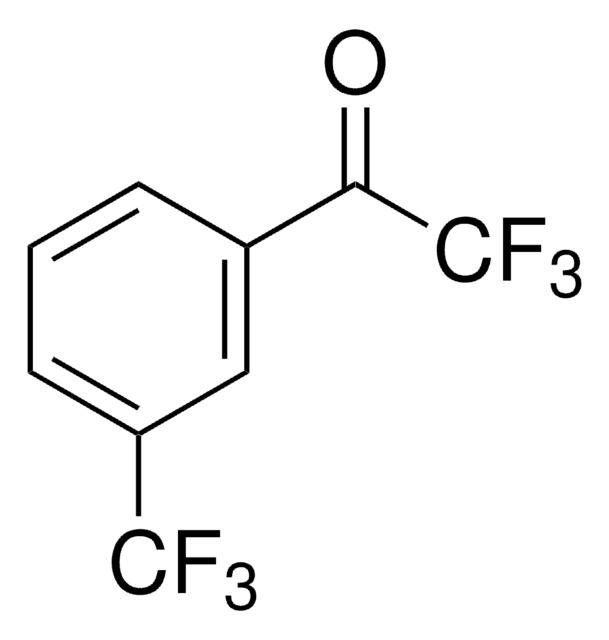
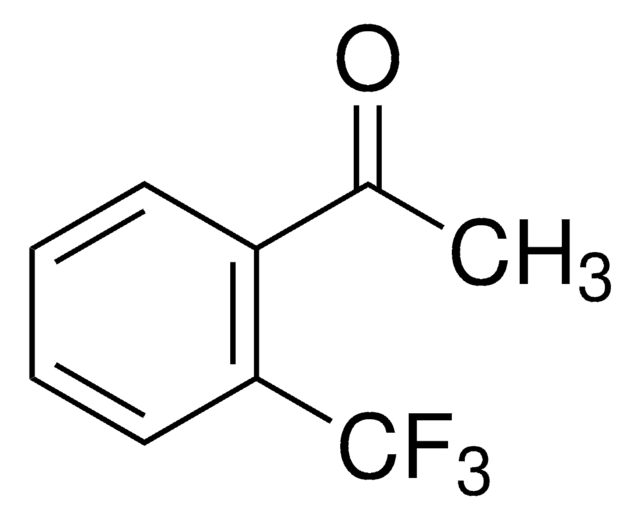
2,2,2-Trifluoroacetophenone
99%
Synonym(s):
α,α,α-Trifluoroacetophenone, Phenyl trifluoromethyl ketone
About This Item
Recommended Products
Quality Level
assay
99%
refractive index
n20/D 1.458 (lit.)
bp
165-166 °C (lit.)
46-48 °C/14 mmHg (lit.)
density
1.24 g/mL at 25 °C (lit.)
functional group
fluoro
ketone
phenyl
SMILES string
FC(F)(F)C(=O)c1ccccc1
InChI
1S/C8H5F3O/c9-8(10,11)7(12)6-4-2-1-3-5-6/h1-5H
InChI key
KZJRKRQSDZGHEC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,2,2-trifluoroacetophenone is used as an organocatalyst for the oxidation of tertiary amines and azines to N-oxidesalkenes.
Application
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
105.8 °F - closed cup
flash_point_c
41 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 107840-100G | 4061838675859 |
| 107840-25G | 4061838675873 |
| 107840-5G | 4061838675880 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service