419982
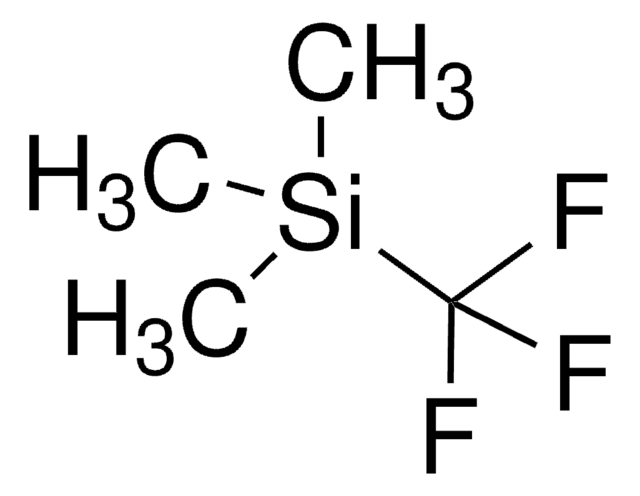
Triethyl(trifluoromethyl)silane
98%
Synonym(s):
(Trifluoromethyl)triethylsilane, Triethylsilyl trifluoromethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3SiCF3
CAS Number:
Molecular Weight:
184.27
Beilstein/REAXYS Number:
4242161
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
reaction suitability
reaction type: C-C Bond Formation
refractive index
n20/D 1.382 (lit.)
bp
56-57 °C/60 mmHg (lit.)
density
0.98 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
CC[Si](CC)(CC)C(F)(F)F
InChI
1S/C7H15F3Si/c1-4-11(5-2,6-3)7(8,9)10/h4-6H2,1-3H3
InChI key
ZHSKFONQCREGOG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
- Trifluoromethylation of aryl iodides
- Trialkylsilylation reactions
- Used for deposition of perfluoro-methyl silica films
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eun Jin Cho et al.
Science (New York, N.Y.), 328(5986), 1679-1681 (2010-06-26)
The trifluoromethyl group can dramatically influence the properties of organic molecules, thereby increasing their applicability as pharmaceuticals, agrochemicals, or building blocks for organic materials. Despite the importance of this substituent, no general method exists for its installment onto functionalized aromatic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service