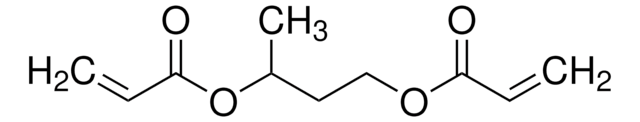
411744
1,4-Butanediol diacrylate
technical grade, contains ~75 ppm hydroquinone as inhibitor
Synonym(s):
1,4-Bis(acryloyloxy)butane, Tetramethylene diacrylate
About This Item
Recommended Products
grade
technical grade
assay
87%
form
liquid
contains
~75 ppm hydroquinone as inhibitor
refractive index
n20/D 1.456 (lit.)
bp
83 °C/0.3 mmHg (lit.)
density
1.051 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC(=O)OCCCCOC(=O)C=C
InChI
1S/C10H14O4/c1-3-9(11)13-7-5-6-8-14-10(12)4-2/h3-4H,1-2,5-8H2
InChI key
JHWGFJBTMHEZME-UHFFFAOYSA-N
General description
Application
- As a precursor to synthesize joint-linker hydrogels with good mechanical strength and used as scaffold materials in bone tissue engineering as biomimetics for natural tissues and also in drug delivery systems.
- To prepare anti-fouling coating for dental composites.
- As a crosslinking agent to prepare hydrophobic acrylic intraocular lens(IOL) materials with reduced glistening.
- As a precursor to fabricate poly(β-amino ester) based solid polymer electrolytefilms for Li-ion batteries. BDDA enhances the ionic conductivity of theelectrolyte films.
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
>235.4 °F
flash_point_c
> 113 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Discussion of synthetic modifications to gelatin, improving the three-dimensional (3D) print resolution, and resulting material properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service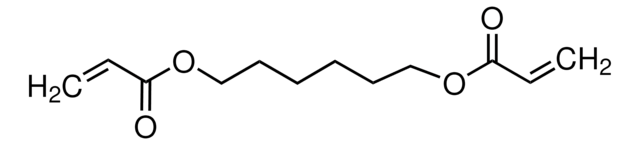
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)