381438
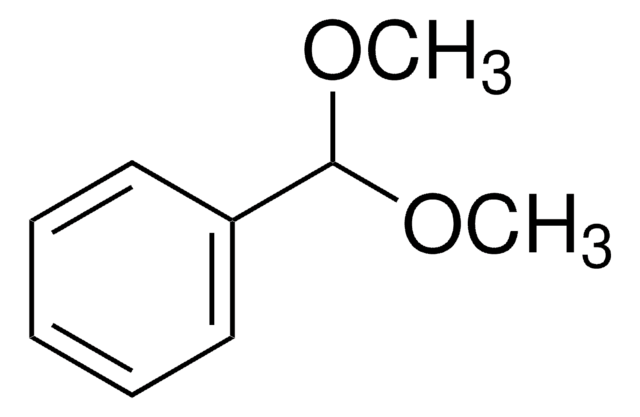
Benzaldehyde dimethyl acetal
95%
Synonym(s):
α,α-Dimethoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(OCH3)2
CAS Number:
Molecular Weight:
152.19
Beilstein/REAXYS Number:
2044501
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
refractive index
n20/D 1.493 (lit.)
bp
87-89 °C/18 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
functional group
acetal
ether
phenyl
SMILES string
COC(OC)c1ccccc1
InChI
1S/C9H12O2/c1-10-9(11-2)8-6-4-3-5-7-8/h3-7,9H,1-2H3
InChI key
HEVMDQBCAHEHDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
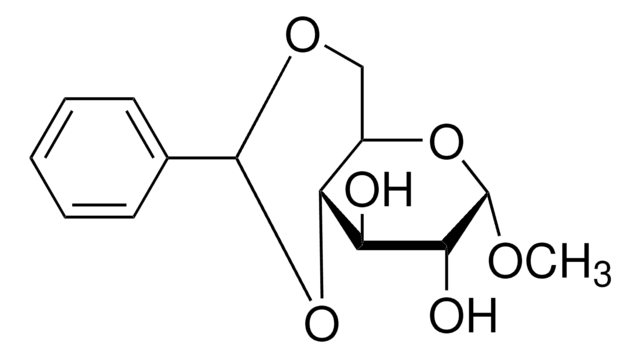
Benzaldehyde dimethyl acetal is an organic building block. Pyridinium tosylate-catalyzed acetal exchange reaction between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-α-D-glucofuranose is reported to afford 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranose. The kinetics of the hydrolysis of benzaldehyde dimethyl acetal over amberlite IR-120 has been studied using a circulated batch reactor in dioxane. One-pot tandem conversion of benzaldehydedimethylacetal to trans-1-nitro-2-phenylethylene has been reported.
Application
Benzaldehyde dimethyl acetal is suitable for use in the synthesis of 4,6-dihydroxy sugar, required for the total synthesis of Porphyromonas gingivalis 381 derived lipid A. It may be used in the preparation of 1-O-methyl-2,3-di-O-galloyl-β-D-glucose.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
156.2 °F - closed cup
flash_point_c
69 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Akerfeldt et al.
Carbohydrate research, 158, 137-145 (1986-12-15)
Pyridinium tosylate-catalyzed acetal exchange between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-alpha-D-glucofu ranose was investigated as an alternative to the original procedure of Brigl and Grüner (condensation of a D-glucose triol with benzaldehyde under zinc halide catalysis) for synthesis of 3,5-O-benzylidene-1,2-O-isopropylidene-alpha-D-glucofuranose. The
T Ogawa et al.
FEMS immunology and medical microbiology, 28(4), 273-281 (2000-07-13)
A synthetic lipid A of Porphyromonas gingivalis strain 381 (compound PG-381), which is similar to its natural lipid A, demonstrated no or very low endotoxic activities as compared to Escherichia coli-type synthetic lipid A (compound 506). On the other hand
M Toda et al.
Bioscience, biotechnology, and biochemistry, 65(3), 542-547 (2001-05-02)
The clove ellagitannins and their related polygalloyl-glucoses inhibited maltase activity of rat intestinal alpha-glucosidases. The structure-activity relationship study of those galloylglucoses, varying the extent of galloylation on the glucose core, with the ellagitannins, indicated that an increasing number of galloyl
Mesoporous silica with site-isolated amine and phosphotungstic acid groups: a solid catalyst with tunable antagonistic functions for one-pot tandem reactions.
N Raveendran Shiju et al.
Angewandte Chemie (International ed. in English), 50(41), 9615-9619 (2011-09-16)
Kinetics of hydrolysis of benzaldehyde dimethyl acetal over Amberlite IR-120.
Altiokka MR and Hosgun HL.
Industrial & Engineering Chemistry Research, 46(4), 1058-1062 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service