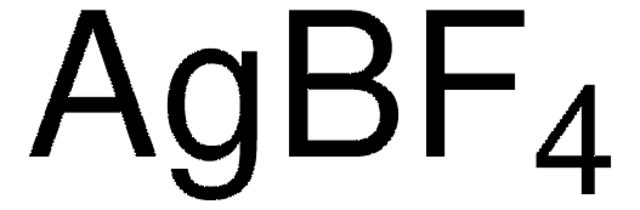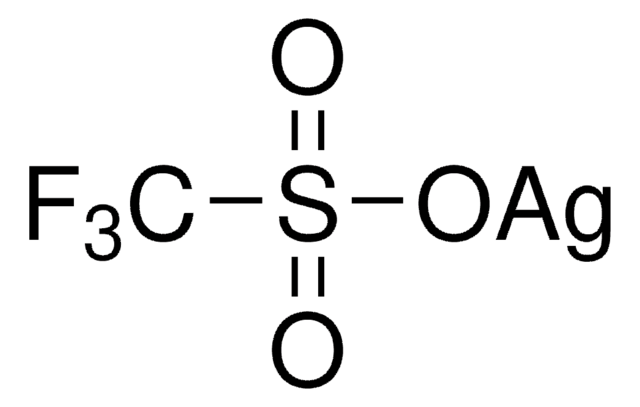176435
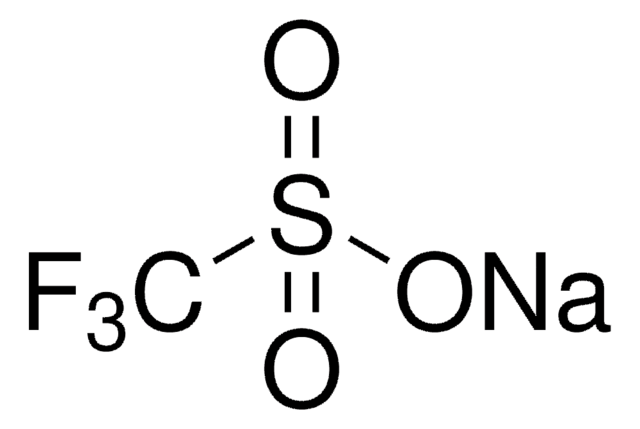
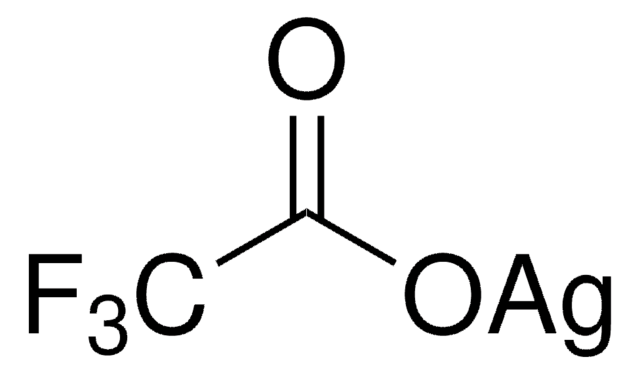
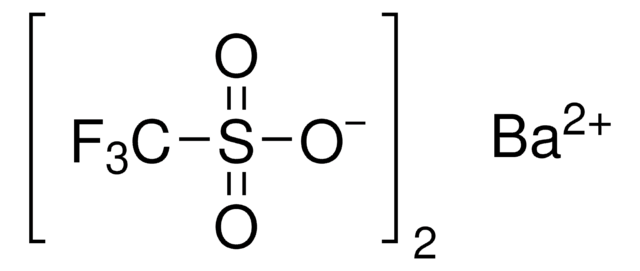
Silver trifluoromethanesulfonate
≥99%
Synonym(s):
Silver triflate, Trifluoromethanesulfonic acid silver salt
About This Item
Recommended Products
Quality Level
assay
≥99%
form
powder
reaction suitability
core: silver
reagent type: catalyst
mp
286 °C (lit.)
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
It can also be used:
- To obtain olefins from secondary phosphates and thiophosphates.
- As a reagent in the etherification of alcohols with primary alkyl halides under mild conditions.
- To generate cationic rhodium catalysts from chlororhodium complexes for the hydrophosphination of acetylenes.
- As a catalyst for the preparation of silyl ethers by hydrosilylation of aldehydes.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service