All Photos(2)
About This Item
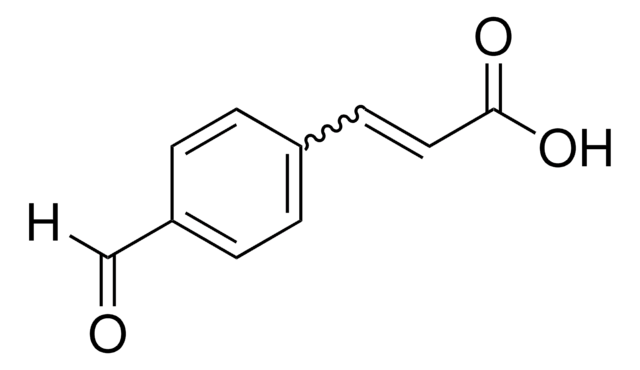
Linear Formula:
BrC6H4CH=CHCO2H
CAS Number:
Molecular Weight:
227.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
262-264 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
OC(=O)\C=C\c1ccc(Br)cc1
InChI
1S/C9H7BrO2/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-6H,(H,11,12)/b6-3+
InChI key
CPDDDTNAMBSPRN-ZZXKWVIFSA-N
Related Categories
Application
4-Bromocinnamic acid (trans-4-bromocinnamic acid) has been used in the preparation of:
- (E)-β-bromo-4-bromostyrene
- 2-amino-7-(piperidin-4-yl)isoquinoline
- brominated dansyl derivative (4-bromophenyl)-4-(5-(dimethylamino)naphthalene-1-sulfonamido)butanoic acid
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, synthesis, and biological evaluation of a dansyled amino acid derivative as an imaging agent for apoptosis.
Zeng W, et al.
Tetrahedron Letters, 49(45), 6429-6432 (2008)
A Convenient Synthesis of 1-Amino-7-(Piperidin-4-yl) Isoquinoline.
Shkavrov S, et al.
Synthetic Communications, 35(5), 725-730 (2005)
Stereoselective synthesis of (E)-?-arylvinyl bromides by microwave-induced reaction of anti-3-aryl-2, 3-dibromopropanoic acids using an AgOAc-AcOH system.
Kuang C, et al.
Tetrahedron, 61(3), 637-642 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service