241571
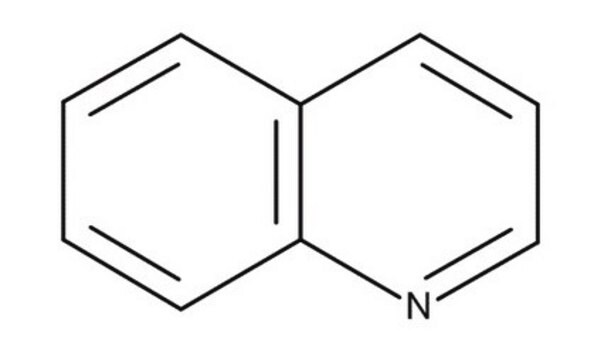
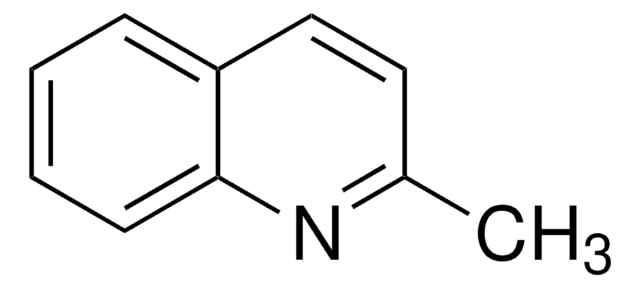
Quinoline
reagent grade, 98%
Synonym(s):
1-Benzazine, 2,3-Benzopyridine
About This Item
Recommended Products
grade
reagent grade
Quality Level
vapor density
4.5 (vs air)
vapor pressure
0.07 mmHg ( 20 °C)
assay
98%
form
liquid
autoignition temp.
896 °F
refractive index
n20/D 1.625 (lit.)
bp
113-114 °C/11 mmHg (lit.)
237 °C (lit.)
mp
−17-−13 °C (lit.)
density
1.093 g/mL at 25 °C (lit.)
SMILES string
c1ccc2ncccc2c1
InChI
1S/C9H7N/c1-2-6-9-8(4-1)5-3-7-10-9/h1-7H
InChI key
SMWDFEZZVXVKRB-UHFFFAOYSA-N
Gene Information
human ... CYP2D6(1565)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Disclaimer
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Chronic 2 - Carc. 1B - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
213.8 °F - closed cup
flash_point_c
101 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service