All Photos(1)
About This Item
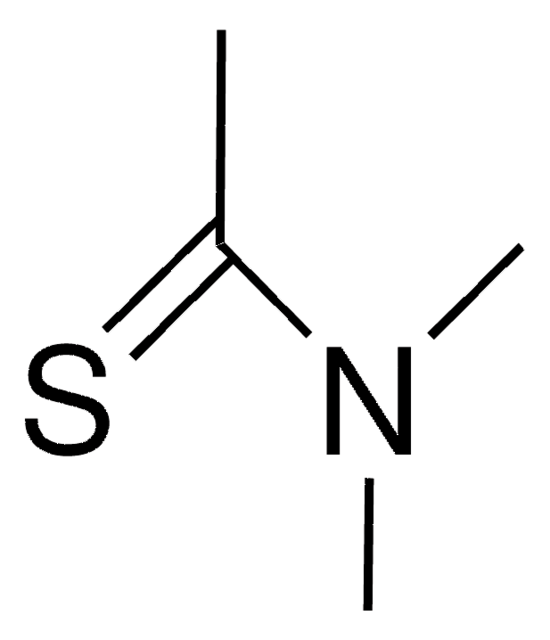
Linear Formula:
HCSN(CH3)2
CAS Number:
Molecular Weight:
89.16
Beilstein/REAXYS Number:
1737191
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98%
form
liquid
refractive index
n20/D 1.576 (lit.)
bp
58-60 °C/1 mmHg (lit.)
density
1.047 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
[H]C(=S)N(C)C
InChI
1S/C3H7NS/c1-4(2)3-5/h3H,1-2H3
InChI key
SKECXRFZFFAANN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N,N-Dimethylthioformamide undergoes desulfurization reaction with hydrosilane under photo irradiation in the presence of a methyl iron complex. It forms a deep-orange complex with anhydrous bismuth(III) trifluoromethanesulfonate.
Application
N,N-Dimethylthioformamide was used as sulfur donor solvent to investigate the coordination chemistry of lead(II).
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
210.2 °F - closed cup
flash_point_c
99 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kozo Fukumoto et al.
Chemical communications (Cambridge, England), 48(32), 3809-3811 (2012-01-06)
Desulfurization of N,N-dimethylthioformamide (Me(2)NCHS) by hydrosilane has been achieved under photo irradiation in the presence of a methyl iron complex. The reaction sequences have been proposed, in which silyl migration from Fe to S of thioformamide triggers the cleavage of
Krzysztof Lyczko et al.
Inorganic chemistry, 43(22), 7094-7100 (2004-10-27)
At the dissolution of anhydrous bismuth(III) trifluoromethanesulfonate in N,N-dimethylthioformamide (DMTF) a deep red-orange complex, lambda(max) = 457 nm, is formed. Bismuth(III) is reduced by the solvent to a low-valent oxidation state stabilized by the sulfur-coordinating solvent DMTF. The obtained complex
Ingmar Persson et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 22(51), 18583-18592 (2016-11-20)
The coordination chemistry of d10 s2 metal ions is strongly affected by an (at least partially) occupied d10 s2 metal ion-ligand atom antibonding orbital, which may cause a void in the coordination sphere due to repulsion between the electrons in
Ingmar Persson et al.
Inorganic chemistry, 50(3), 1058-1072 (2011-01-14)
The coordination chemistry of lead(II) in the oxygen donor solvents water, dimethylsulfoxide (dmso, Me(2)SO), N,N-dimethylformamide (dmf), N,N-dimethylacetamide (dma), N,N'-dimethylpropyleneurea (dmpu), and 1,1,3,3-tetramethylurea (tmu), as well as in the sulfur donor solvent N,N-dimethylthioformamide (dmtf), has been investigated by extended X-ray absorption
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service