10919
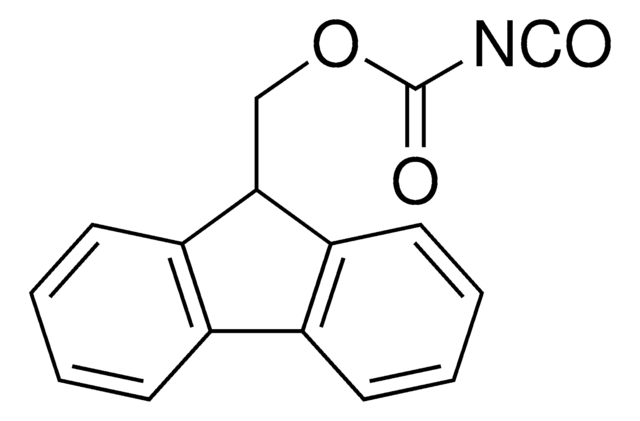
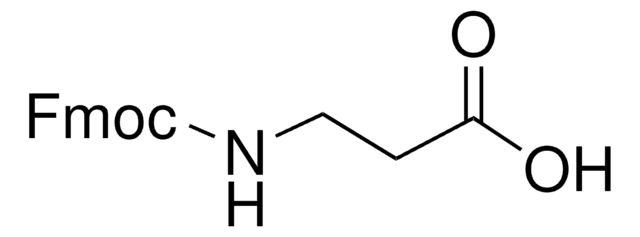
Fmoc isothiocyanate
≥98.0% (CHN)
Synonym(s):
9-Fluorenylmethoxycarbonyl isothiocyanate, 9-Fluorenylmethyl isothiocyanatoformate
About This Item
Recommended Products
Quality Level
assay
≥85% (coupling to amines)
≥98.0% (CHN)
solubility
ethanol: soluble
fluorescence
λex 264 nm; λem 313 nm
application(s)
peptide synthesis
functional group
Fmoc
amine
isothiocyanate
storage temp.
−20°C
SMILES string
O=C(OCC1c2ccccc2-c3ccccc13)N=C=S
InChI
1S/C16H11NO2S/c18-16(17-10-20)19-9-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9H2
InChI key
DHMYULZVFHHEHE-UHFFFAOYSA-N
Application
- As a starting material in the preparation of biologically relevant pharmacophores named N-aryl-N′-carboalkoxy guanidines.
- In one of the intermediate steps for the synthesis of N-aryl-N-thiazolyl derivatives.
- To synthesize cyclic isothiourea derivatives as potent neuropeptide Y (NPY) Y1 receptor antagonists.
- To prepare 2-aminothiazoles, aminobenz-imidazole conjugated thiazoles, and thiazole derived cyclopeptides.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service