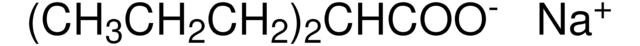推薦產品
等級
pharmaceutical primary standard
API 家族
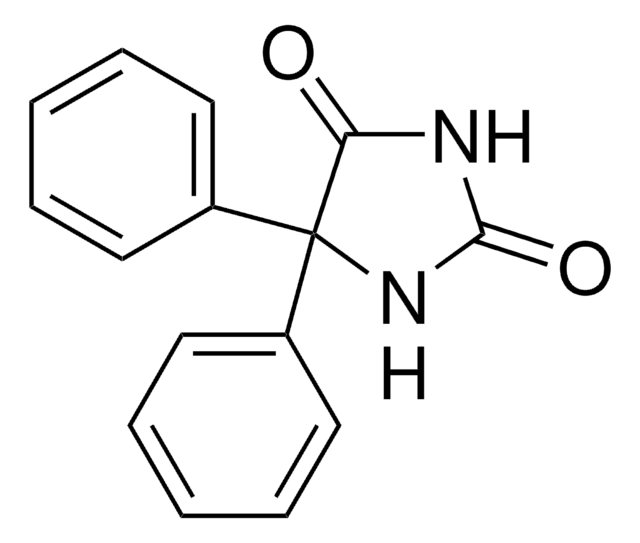
phenytoin
製造商/商標名
USP
mp
293-295 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
O=C1NC(=O)C(N1)(c2ccccc2)c3ccccc3
InChI
1S/C15H12N2O2/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19)
InChI 密鑰
CXOFVDLJLONNDW-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Phenytoin USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Phenytoin Sodium
- Phenytoin Chewable Tablets
- Phenytoin Oral Suspension
- Phenytoin Sodium Injection
- Extended Phenytoin Sodium Capsules
- Phenytoin Compounded Topical Gel
- Fosphenytoin Sodium Injection
生化/生理作用
减少癫痫大发作的发生率;可能通过 Na+、K+ 和 Ca2+ 通道的影响而稳定可兴奋膜。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 2 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
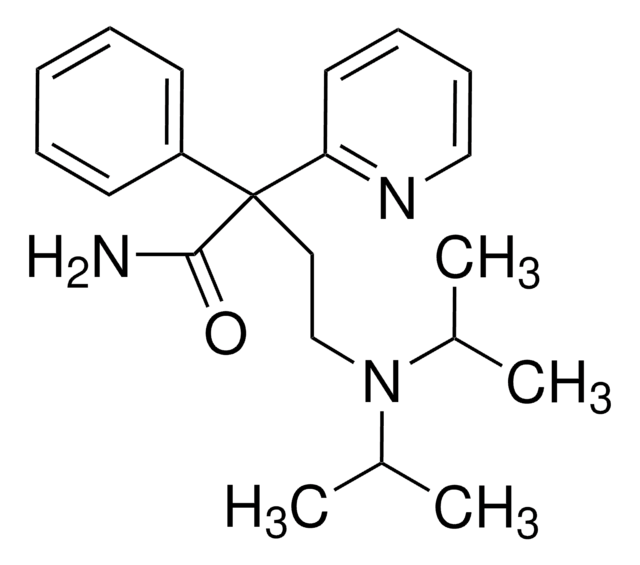
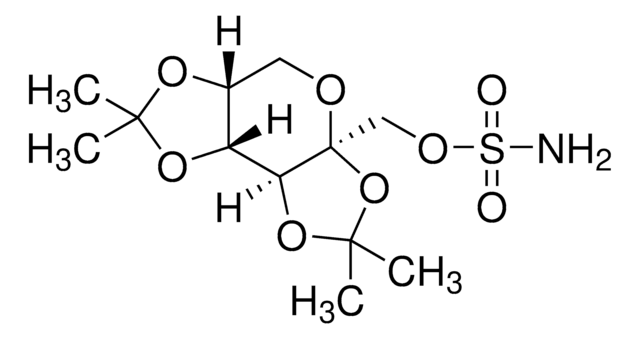
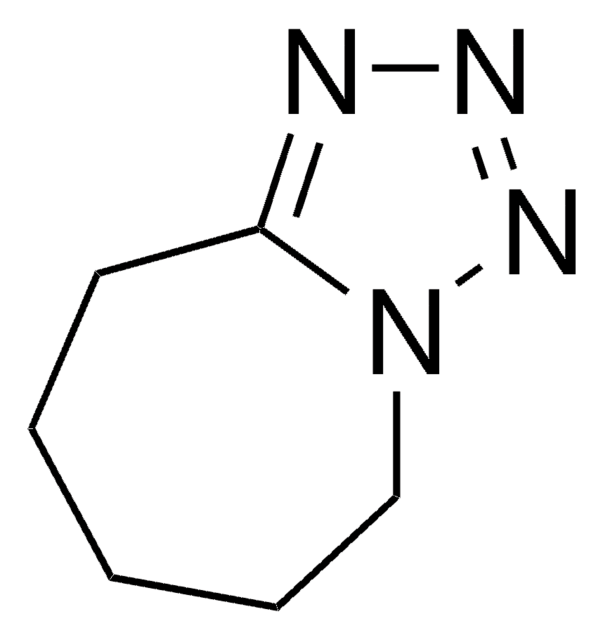
客戶也查看了
Deborah Pugin et al.
Critical care (London, England), 18(3), R103-R103 (2014-06-03)
Seizures refractory to third-line therapy are also labeled super-refractory status epilepticus (SRSE). These seizures are extremely difficult to control and associated with poor outcome. We aimed to characterize efficacy and side-effects of continuous infusions of pentobarbital (cIV-PTB) treating SRSE. We
Chunhong Shen et al.
Bone, 64, 246-253 (2014-05-02)
It has been shown that antiepileptic drugs (AEDs) may have a detrimental effect on bone health and translate into an increased risk of bone fracture. We aimed to comprehensively evaluate the association between use of AEDs and fracture risk. We
Syed Nabeel Zafar et al.
BMC neurology, 12, 30-30 (2012-05-31)
Current standard therapy for seizure prophylaxis in Neuro-surgical patients involves the use of Phenytoin (PHY). However, a new drug Levetiracetam (LEV) is emerging as an alternate treatment choice. We aimed to conduct a meta-analysis to compare these two drugs in
Lelia Duley et al.
The Cochrane database of systematic reviews, (10)(10), CD000128-CD000128 (2010-10-12)
Eclampsia, the occurrence of a seizure in association with pre-eclampsia, remains a rare but serious complication of pregnancy. A number of different anticonvulsants have been used to control eclamptic fits and to prevent further seizures. The objective of this review
P J Smith et al.
Endocrine reviews, 5(4), 514-524 (1984-01-01)
The studies described above indicate the likelihood of a significant effect of DPH on cellular functions that are regulated by T3 at concentrations of DPH that occur during treatment of patients with Dilantin. Thus, it is possible that sensitive measures
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務