推薦產品
等級
pharmaceutical primary standard
API 家族
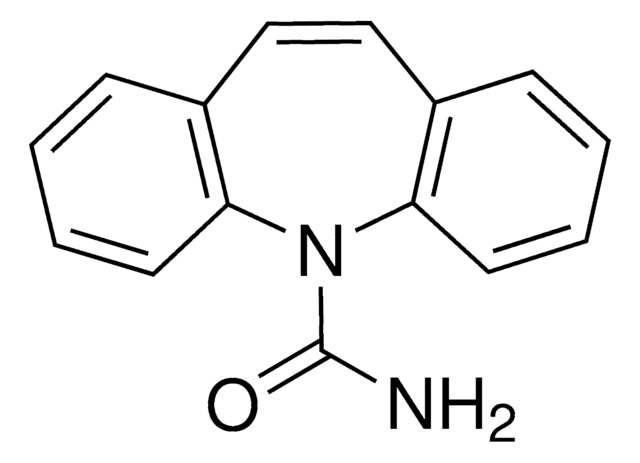
carbamazepine
製造商/商標名
USP
mp
191-192 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
NC(=O)N1c2ccccc2C=Cc3ccccc13
InChI
1S/C15H12N2O/c16-15(18)17-13-7-3-1-5-11(13)9-10-12-6-2-4-8-14(12)17/h1-10H,(H2,16,18)
InChI 密鑰
FFGPTBGBLSHEPO-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Carbamazepine USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Carbamazepine Extended-Release Tablets
- Carbamazepine Oral Suspension
- Carbamazepine Tablets
- Oxcarbazepine
- Oxcarbazepine Oral Suspension
- Oxcarbazepine Tablets
生化/生理作用
抗惊厥药;GABAA受体苯二氮卓调节位点的配体。钠离子通道抑制剂。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Repr. 1A - Resp. Sens. 1 - Skin Sens. 1A - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Anna Jurado et al.
Chemosphere, 115, 47-53 (2014-02-25)
This paper deals with urban groundwater contaminated with carbamazepine (CBZ) and five of its human metabolites in Barcelona. Groundwater samples were accordingly collected in the aquifers of Poble Sec and Besòs River Delta. Higher concentrations and more compounds were found
Philip J Wiffen et al.
The Cochrane database of systematic reviews, (1)(1), CD005451-CD005451 (2011-01-21)
Carbamazepine is used to treat chronic neuropathic pain. Evaluation of analgesic efficacy and adverse effects of carbamazepine for acute and chronic pain management (except headaches). Randomised controlled trials (RCTs) of carbamazepine in acute, chronic or cancer pain were identified, searching
Wimonchat Tangamornsuksan et al.
JAMA dermatology, 149(9), 1025-1032 (2013-07-26)
The US Food and Drug Administration recommends screening for the HLA-B*1502 allele before initiation of carbamazepine therapy in patients of Asian ancestry, but there remains unclear evidence of a relationship between HLA-B*1502 and Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Ursula Amstutz et al.
Epilepsia, 55(4), 496-506 (2014-03-07)
To systematically review evidence on genetic risk factors for carbamazepine (CBZ)-induced hypersensitivity reactions (HSRs) and provide practice recommendations addressing the key questions: (1) Should genetic testing for HLA-B*15:02 and HLA-A*31:01 be performed in patients with an indication for CBZ therapy
Sandeep Grover et al.
Pharmacogenetics and genomics, 24(2), 94-112 (2013-12-18)
A considerable heterogeneity exists in the literature on the role of different HLA alleles in carbamazepine (CBZ)-induced cutaneous adverse drug reactions (cADRs) of varying severity among diverse ethnic groups. The aim of the present study was to understand and summarize
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務