推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1093023
API 家族
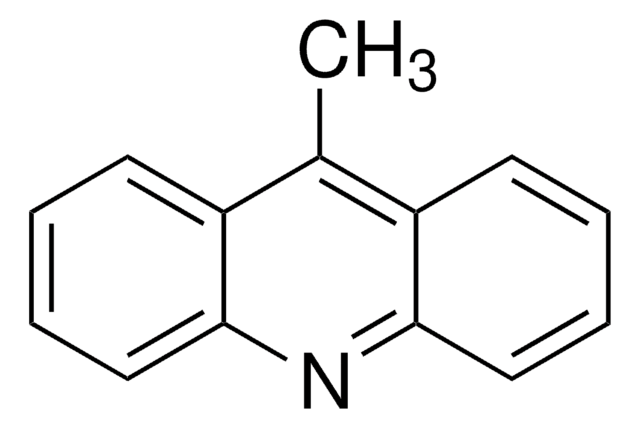
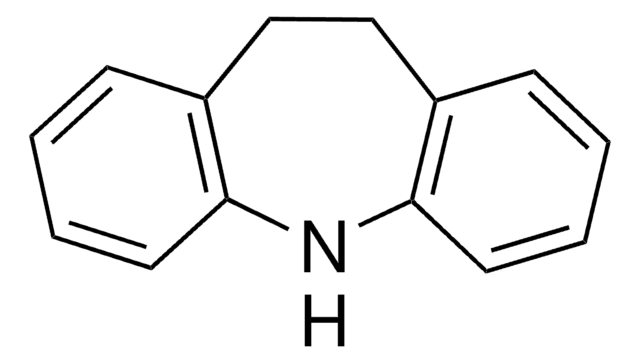
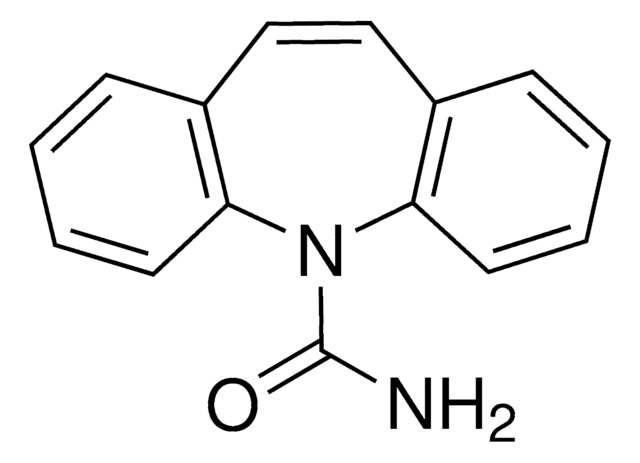
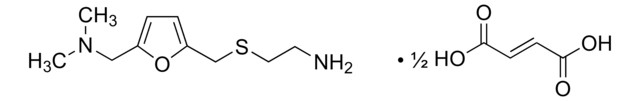
carbamazepine, oxcarbazepine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
196-199 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
N1c2ccccc2C=Cc3ccccc13
InChI
1S/C14H11N/c1-3-7-13-11(5-1)9-10-12-6-2-4-8-14(12)15-13/h1-10,15H
InChI 密鑰
LCGTWRLJTMHIQZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards..
應用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA9013 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
防範說明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Koleta Hemine et al.
Carbohydrate polymers, 250, 116957-116957 (2020-10-15)
It is widely believed that the hydrophobic effect governs the binding of guest molecules to cyclodextrins (CDs). However, it is also known that high hydrophobicity of guest molecules does not always translate to the formation of stable inclusion complexes with
S M Furst et al.
Biochemical pharmacology, 45(6), 1267-1275 (1993-03-24)
Carbamazepine is an anticonvulsant which is associated with a significant incidence of hypersensitivity reactions including agranulocytosis. We have postulated that many drug hypersensitivity reactions, especially agranulocytosis and lupus, are due to reactive metabolites generated by the myeloperoxidase (MPO) (EC 1.11.1.7)
A Varenne et al.
Journal of immunological methods, 186(2), 195-204 (1995-10-26)
As part of our ongoing work to extend the range of applications of the non-isotopic carbonyl metalloimmunoassay (CMIA), previously developed in our laboratory, we describe here the first CMIA study of carbamazepine. The CMIA method uses a metal carbonyl complex
Ying Wu et al.
The Journal of allergy and clinical immunology, 118(1), 233-241 (2006-07-04)
T-cell-mediated hypersensitivity is a rare but serious manifestation of drug therapy. To explore the mechanisms of drug presentation to T cells and the possibility that generation of metabolite-specific T cells may provoke cross-sensitization between drugs. A lymphocyte transformation test was
S M Furst et al.
Drug metabolism and disposition: the biological fate of chemicals, 23(5), 590-594 (1995-05-01)
Carbamazepine therapy is associated with several types of idiosyncratic drug reactions, including hematological disorders. In previous studies, we found that carbamazepine was metabolized by the myeloperoxidase/H2O2 system of activated neutrophils, and covalent binding of the drug to neutrophils was observed.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務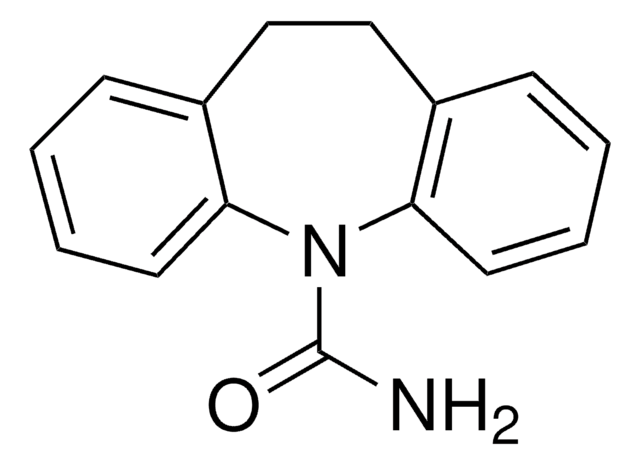
![5H-二苯并[b,f]氮杂 United States Pharmacopeia (USP) Reference Standard](/deepweb/assets/sigmaaldrich/product/structures/396/216/18f00414-a76e-46d7-90cf-820ad902e559/640/18f00414-a76e-46d7-90cf-820ad902e559.png)