全部照片(4)
About This Item
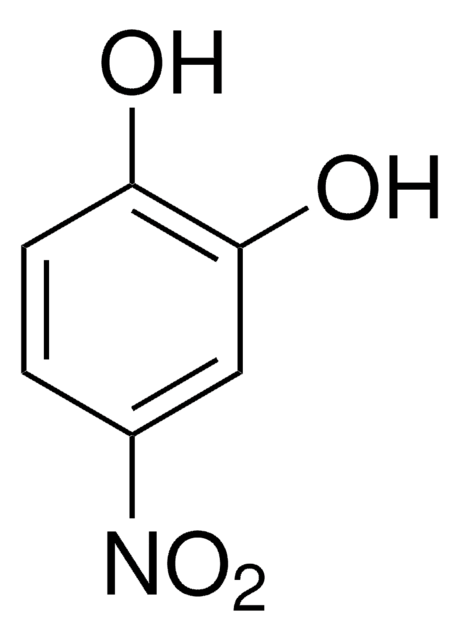
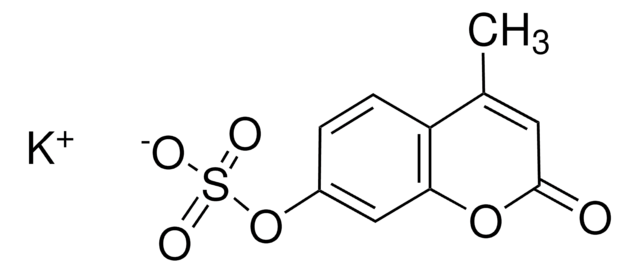
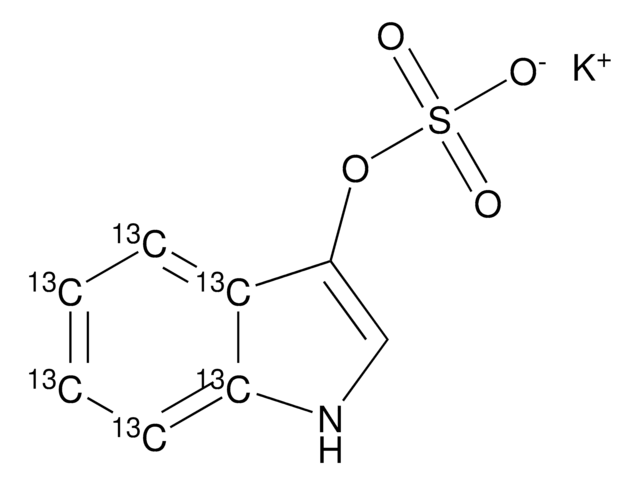
線性公式:
C6H3NO7SK2
CAS號碼:
分子量::
311.35
Beilstein:
3805018
EC號碼:
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
形狀
crystalline
品質等級
顏色
yellow
儲存溫度
−20°C
SMILES 字串
[K+].[K+].[O-]c1ccc(cc1OS([O-])(=O)=O)[N+]([O-])=O
InChI
1S/C6H5NO7S.2K/c8-5-2-1-4(7(9)10)3-6(5)14-15(11,12)13;;/h1-3,8H,(H,11,12,13);;/q;2*+1/p-2
InChI 密鑰
CKWWBDCAYRJAJB-UHFFFAOYSA-L
一般說明
4-硝基儿茶酚硫酸盐是一种芳香族硫酸盐。
應用
4-硝基儿茶酚硫酸二钾盐被用作粘多糖 VI(MPS VI)分析的底物。它也被用来测量糖胺聚糖(GAG)降解酶-芳基磺化酶B和外糖苷酶的活性。
基底
显色硫酸酯酶底物。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
M Lee-Vaupel et al.
Clinica chimica acta; international journal of clinical chemistry, 164(2), 171-180 (1987-04-30)
Arylsulfatase A hydrolyzes the artificial chromogenic substrate 4-nitrocatechol-sulfate at 0 degree C at a rate of 24% of that at 37 degrees C whereas arylsulfatase B is almost inactive at 0 degree C. Based on this observation, a simple assay
M Recksiek et al.
The Journal of biological chemistry, 273(11), 6096-6103 (1998-04-16)
Sulfatases contain an active site formylglycine residue that is generated by post-translational modification. Crystal structures of two lysosomal sulfatases revealed significant similarity to the catalytic site of alkaline phosphatase containing a serine at the position of formylglycine. To elucidate the
Mazdak Khajehpour et al.
Biochemistry, 46(14), 4370-4378 (2007-03-14)
The Yersinia protein tyrosine phosphatase (YopH) contains a loop of ten amino acids (the WPD loop) that covers the entrance of the active site of the enzyme during substrate binding. In this work the substrate mimicking competitive inhibitor p-nitrocatechol sulfate
C O'Fagain et al.
The Biochemical journal, 201(2), 345-352 (1982-02-01)
A simple model is described to account for the anomalous time course of arylsulphatase A. In the case of the ox liver and human placental enzymes the enzyme-nitrocatechol sulphate complex can, in addition to forming products, slowly break down to
S Partanen
The Histochemical journal, 16(5), 501-506 (1984-05-01)
A new, direct-colouring, metal precipitation method for the light microscopical demonstration of arylsulphatases A and B is described. It is based on the reducing capacity of nitrocatechol liberated by arylsulphatases from p-nitrocatechol sulphate. The reaction is carried out in Karnovsky-Roots'
條款
Enzymatic Assay of Sulfatase (EC 3.1.6.1.)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務