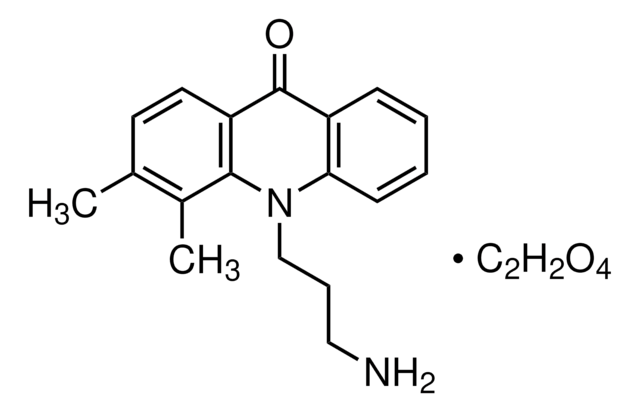
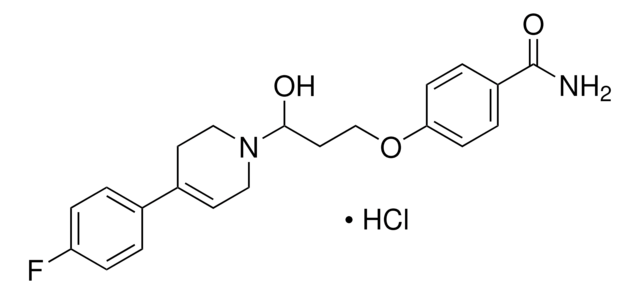
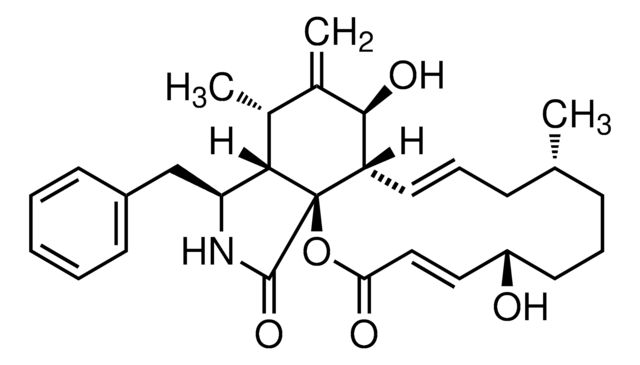
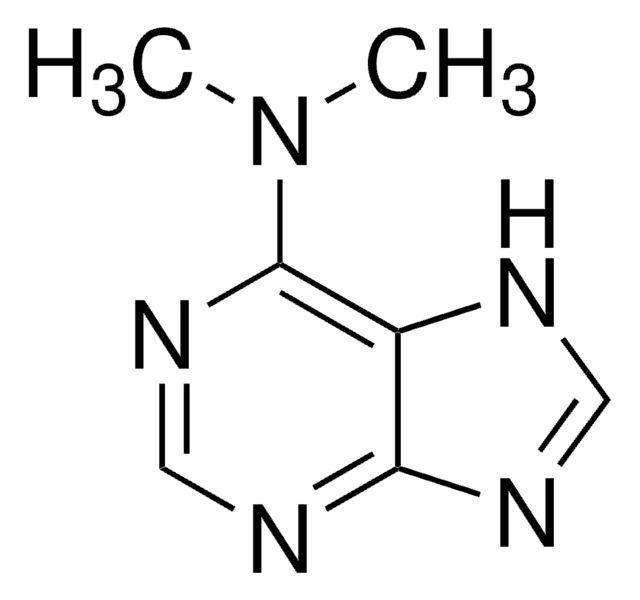
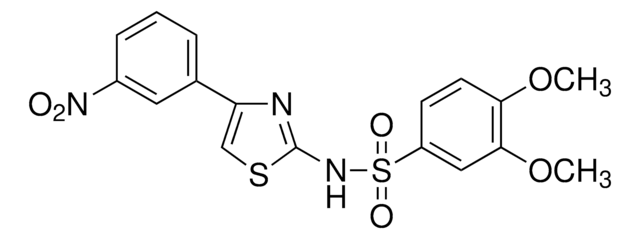
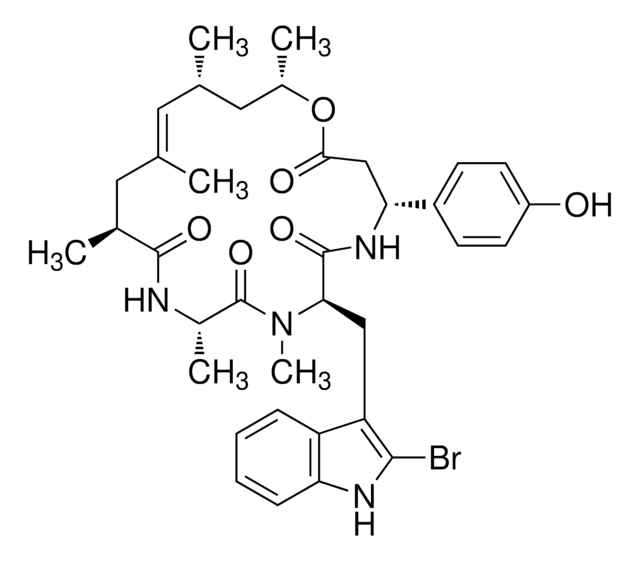
推薦產品
形狀
solid
品質等級
顏色
white
溶解度
H2O: 2 mg/mL
儲存溫度
2-8°C
SMILES 字串
Cl.CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c3ccccc13
InChI
1S/C16H15Cl2N.ClH/c1-19-16-9-13(11-4-2-3-5-12(11)16)10-6-7-14(17)15(18)8-10;/h2-8,13,16,19H,9H2,1H3;1H/t13-,16+;/m0./s1
InChI 密鑰
QICQDZXGZOVTEF-MELYUZJYSA-N
基因資訊
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816) , HTR1A(3350) , HTR1B(3351) , HTR1D(3352) , HTR1E(3354) , HTR1F(3355) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , HTR3A(3359) , HTR3B(9177) , HTR3C(170572) , HTR3D(200909) , HTR3E(285242) , HTR4(3360) , HTR5A(3361) , HTR5B(645694) , HTR6(3362) , HTR7(3363)
應用
- as a competitive inhibitor of 3H-dopamine ([3H]DA) to study its effects on trans-activator of transcription (Tat) protein on cocaine-induced inhibition of uptake of [3H]DA
- as a dopamine transport blocker to study its effects on trace amine-associated receptor 1 (TAAR1)-transfected mice cells
- as a nonselective monoamine transport inhibitor to study its anti-angiogenic activities in glioblastoma multiforme (GBM)
生化/生理作用
特點和優勢
法律資訊
訊號詞
Warning
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Gloves
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務