全部照片(1)
About This Item
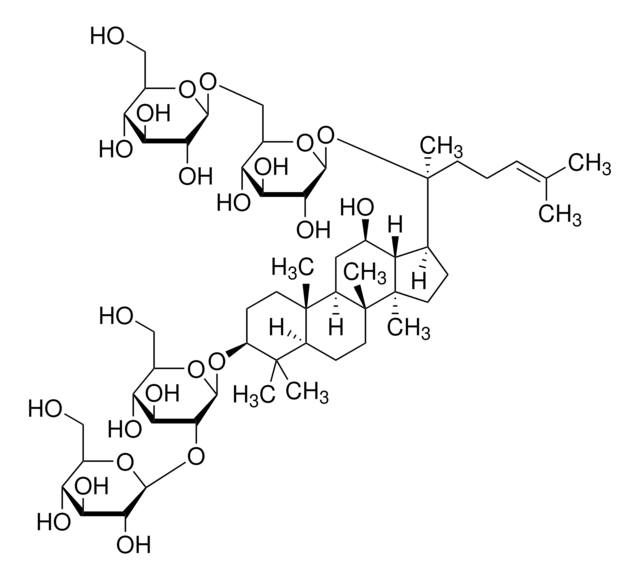
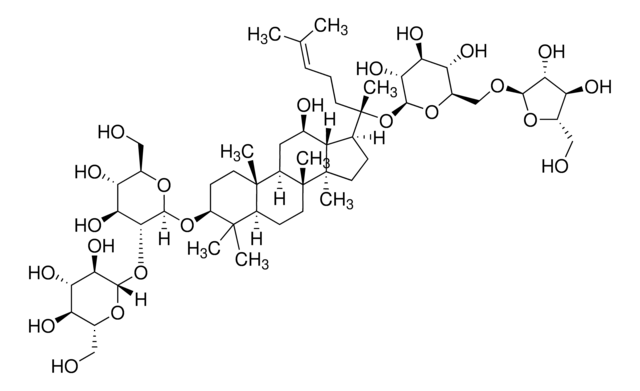
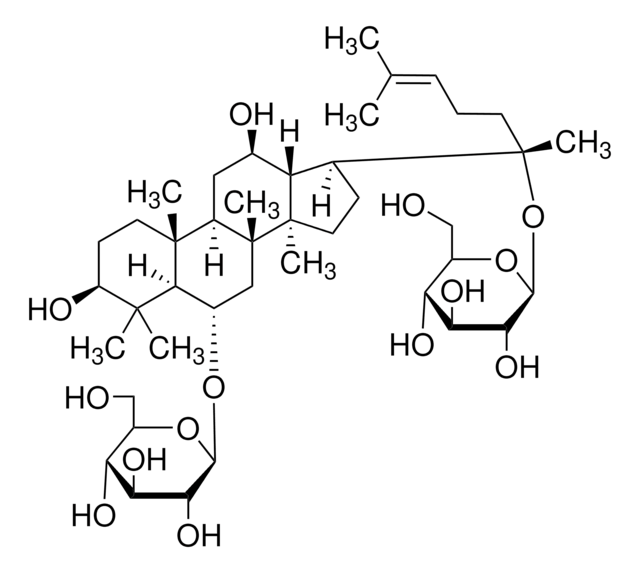
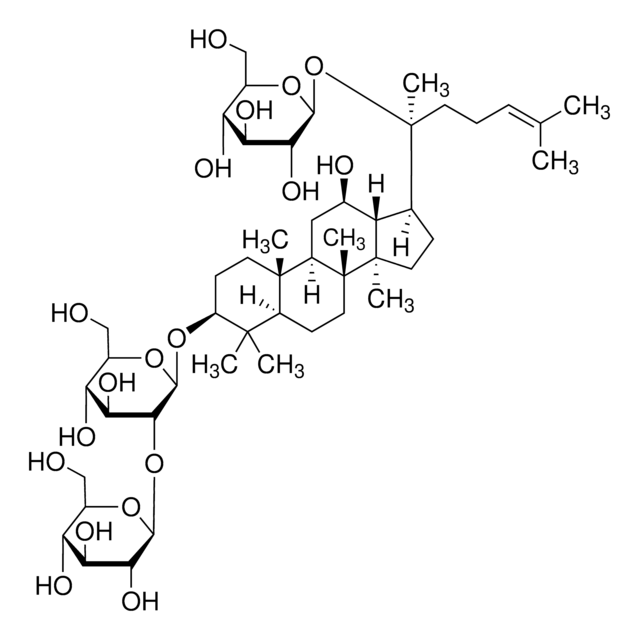
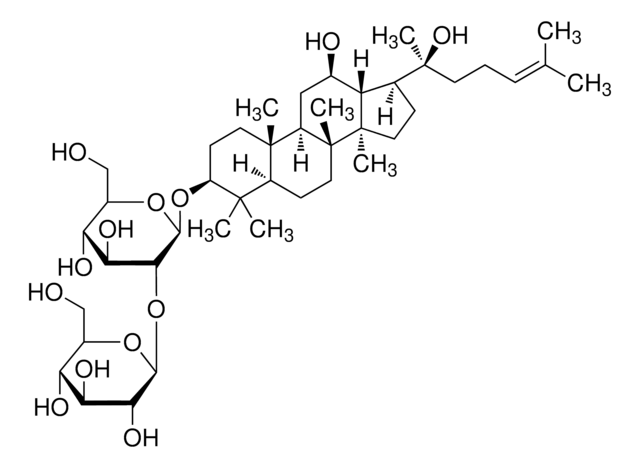
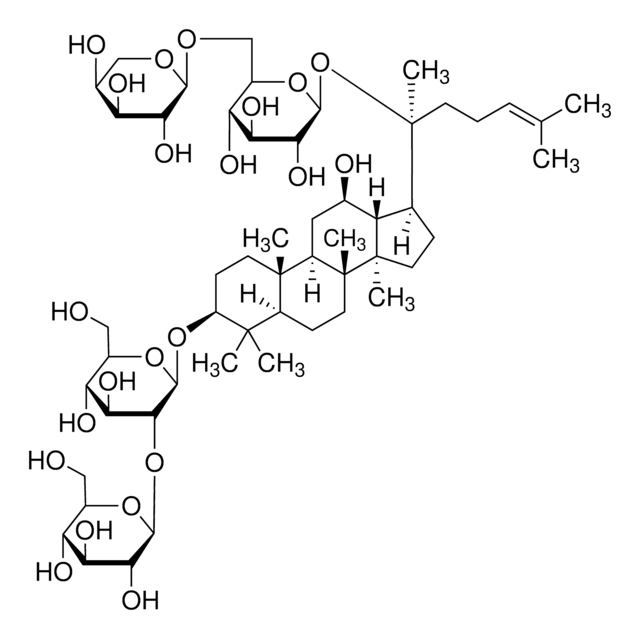
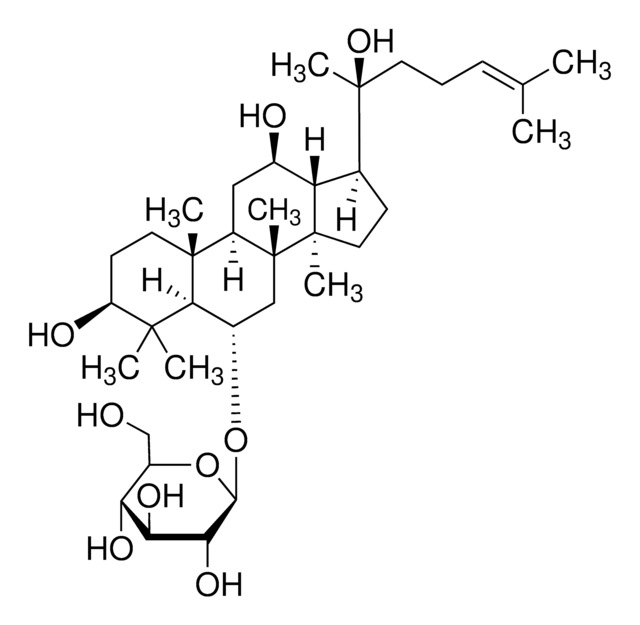
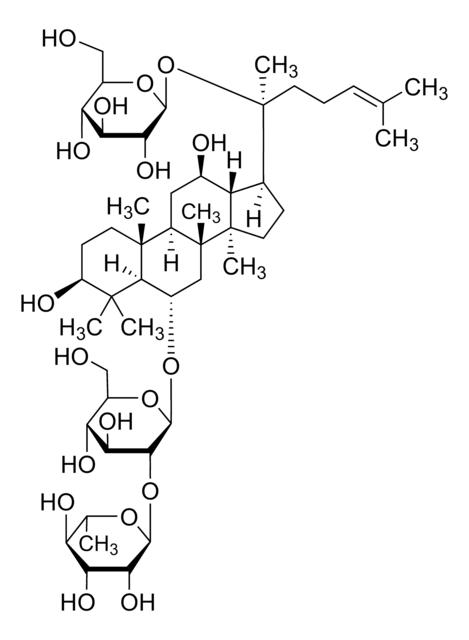
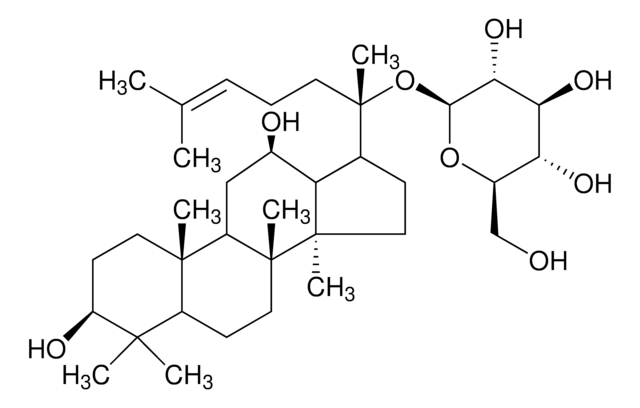
經驗公式(希爾表示法):
C53H90O22
CAS號碼:
分子量::
1079.27
EC號碼:
MDL號碼:
分類程式碼代碼:
12352201
PubChem物質ID:
NACRES:
NA.25
推薦產品
生物源
plant root (Panax ginseng)
品質等級
化驗
≥98% (HPLC)
形狀
powder
技術
HPLC: suitable
儲存溫度
2-8°C
SMILES 字串
C\C(C)=C\CCC(C)(OC1OC(COC2OC(CO)C(O)C2O)C(O)C(O)C1O)C3CCC4(C)C3C(O)CC5C6(C)CCC(OC7OC(CO)C(O)C(O)C7OC8OC(CO)C(O)C(O)C8O)C(C)(C)C6CCC45C
InChI
1S/C53H90O22/c1-23(2)10-9-14-53(8,75-47-43(67)39(63)37(61)29(72-47)22-68-45-41(65)36(60)28(21-56)69-45)24-11-16-52(7)33(24)25(57)18-31-50(5)15-13-32(49(3,4)30(50)12-17-51(31,52)6)73-48-44(40(64)35(59)27(20-55)71-48)74-46-42(66)38(62)34(58)26(19-54)70-46/h10,24-48,54-67H,9,11-22H2,1-8H3
InChI 密鑰
JDCPEKQWFDWQLI-UHFFFAOYSA-N
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Louis Y Chan et al.
Reproductive toxicology (Elmsford, N.Y.), 19(1), 131-134 (2004-09-01)
Pregnant women commonly consume ginseng. However, there is little data concerning the effects of ginseng on early pregnancy. Rat embryos were exposed in vitro to different concentrations of Rc and Re from day 9.5 to day 11.5 after conception. Embryos
Myoung-Su Lee et al.
Journal of ethnopharmacology, 127(3), 771-776 (2009-12-08)
Panax ginseng and its major component, ginsenosides, are widely used for the prevention of various disorders in oriental medicine. To evaluate the effect of ginsenoside Rc (Rc), one of the active constituents in Panax ginseng, on glucose uptake in C2C12
Ron Luchtefeld et al.
Journal of agricultural and food chemistry, 52(16), 4953-4956 (2004-08-05)
A method based on high-performance liquid chromatography (HPLC) and negative ion electrospray mass spectrometry (LC-MS) has been used to determine ginsenosides Rb1, Rc, and Re in six different samples of ginseng. These included a liquid extract, capsules, tea bags, and
T B Ng et al.
Journal of ethnopharmacology, 16(2-3), 191-199 (1986-06-01)
Ginsenosides Rb2, Rc and Rg1 suppressed corticotropin-induced, dibutyryl cyclic AMP-induced and epinephrine-induced lipolysis with the relative potencies Rb2 greater than Rc greater than Rg1. The inhibition of corticotropin-induced lipolysis by ginsenoside Rg1 could not be overcome by increasing the dose
Young Joo Lee et al.
Archives of pharmacal research, 26(1), 53-57 (2003-02-06)
We have found that ginsenoside Rc and Re induce c-fos in MCF-7 human breast carcinoma cells at both the mRNA and protein levels. However, neither ginsenoside activated the expression of reporter gene under the control of AP-1/TPA response elements. We
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務