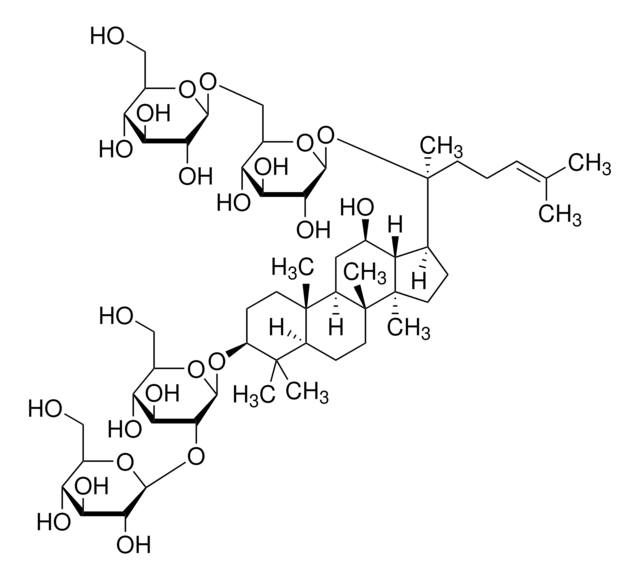
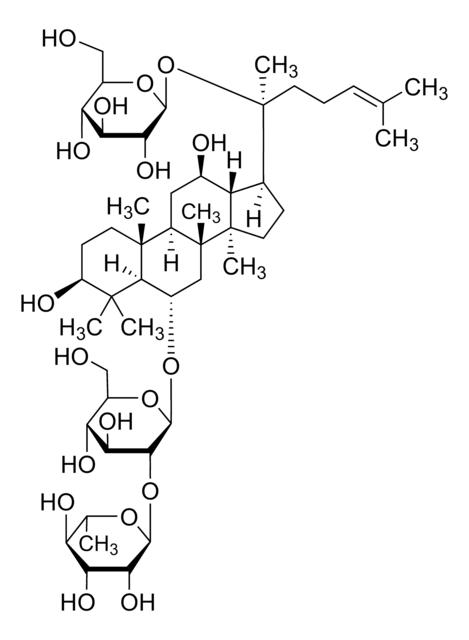
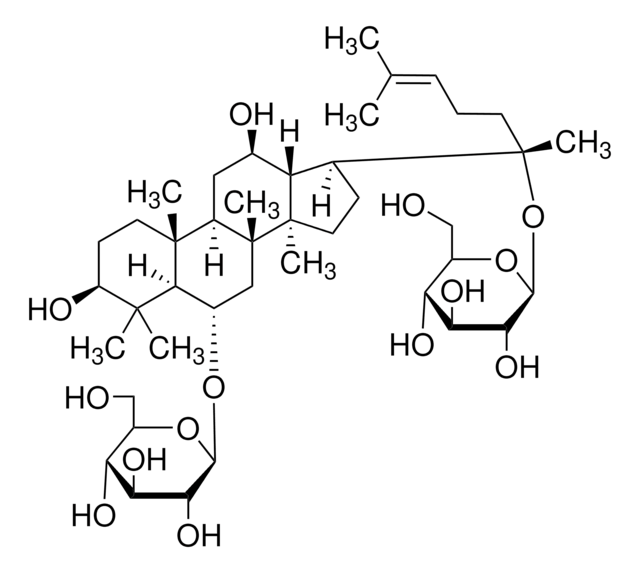
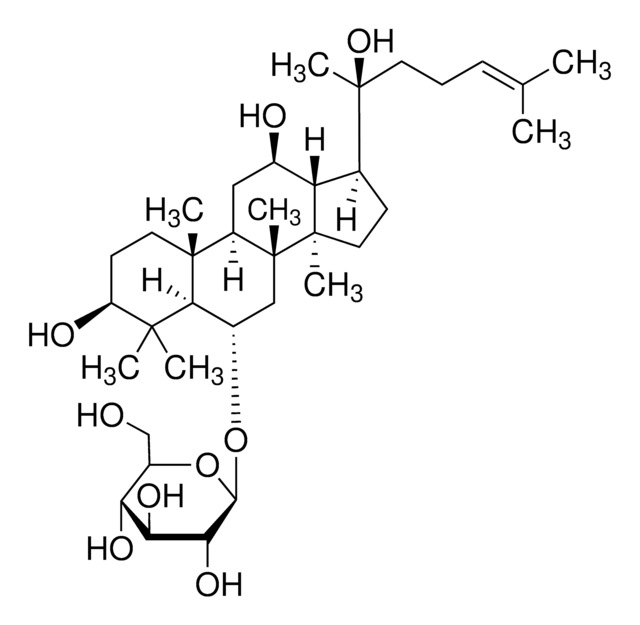
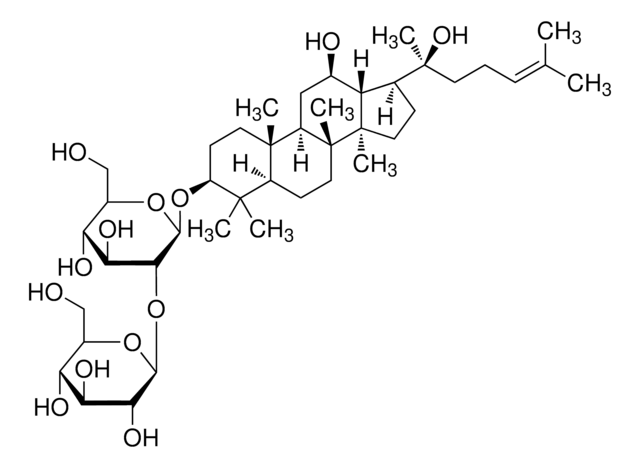
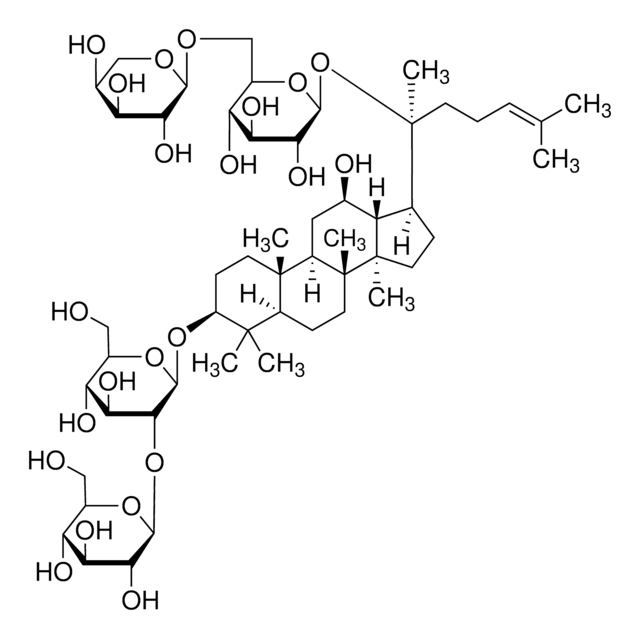
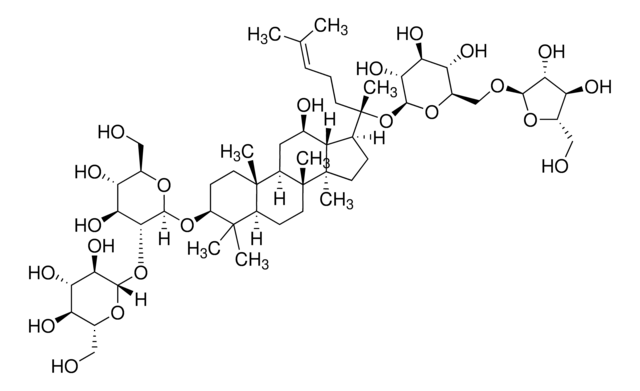
推薦產品
等級
analytical standard
品質等級
化驗
≥90.0% (HPLC)
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
food and beverages
形式
neat
儲存溫度
2-8°C
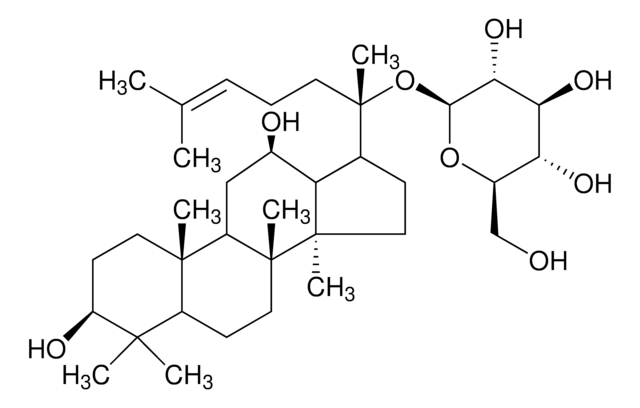
SMILES 字串
C\C(C)=C/CC[C@](C)(O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]3[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4[C@H](C[C@@]23C)O[C@@H]5O[C@H](CO)[C@@H](O)[C@H](O)[C@H]5O
InChI
1S/C36H62O9/c1-19(2)10-9-13-36(8,43)20-11-15-34(6)26(20)21(38)16-24-33(5)14-12-25(39)32(3,4)30(33)22(17-35(24,34)7)44-31-29(42)28(41)27(40)23(18-37)45-31/h10,20-31,37-43H,9,11-18H2,1-8H3/t20-,21+,22-,23+,24+,25-,26-,27+,28-,29+,30-,31+,33+,34+,35+,36-/m0/s1
InChI 密鑰
RAQNTCRNSXYLAH-RFCGZQMISA-N
尋找類似的產品? 前往 產品比較指南
應用
包裝
其他說明
客戶也查看了
文章
In this article we present several HPTLC applications and analytical standards for ginsenosides.
In this article we present several HPTLC applications and analytical standards for ginsenosides.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務