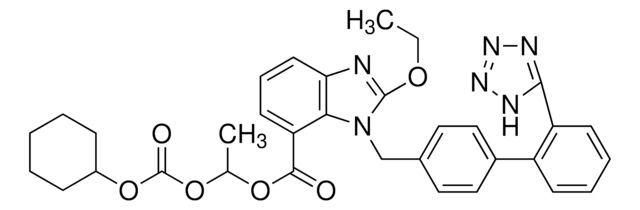
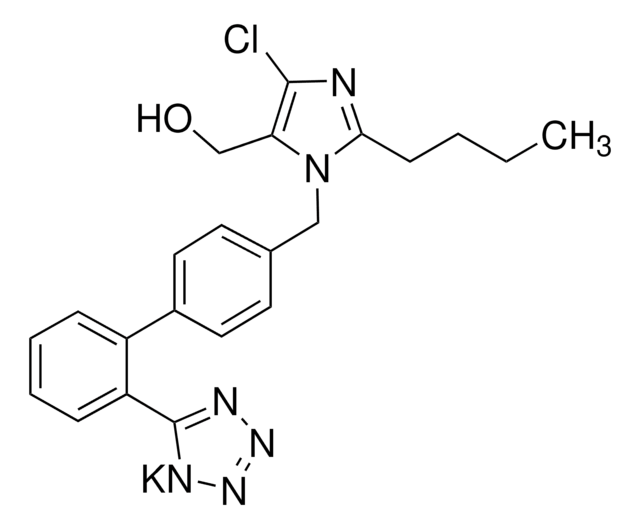
PHR1876
缬沙坦相关化合物B
Pharmaceutical Secondary Standard; Certified Reference Material
同義詞:
N-(1-Oxobutyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine, N-butyryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]methyl}-L-valine, (S)-N-Butyryl-N-{[2′-(1-H-tetrazole-5-yl)-biphenyl-4-yl]methyl}valine
About This Item
推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1708784
API 家族
valsartan
CofA
current certificate can be downloaded
包裝
pkg of 30 mg
應用
pharmaceutical
形式
neat
儲存溫度
2-8°C
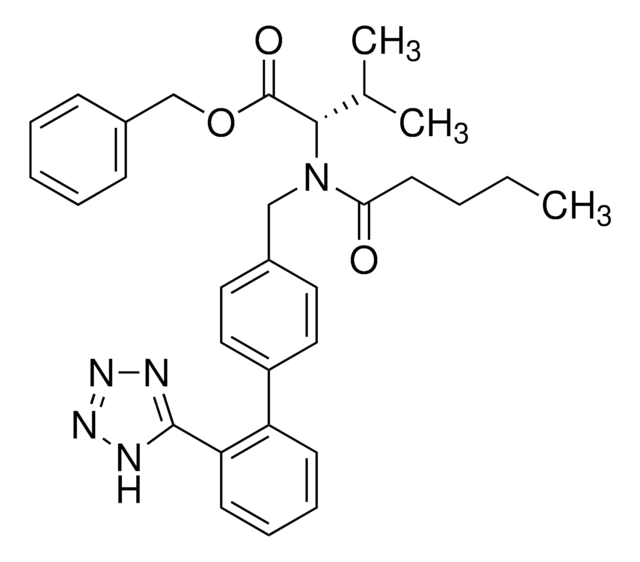
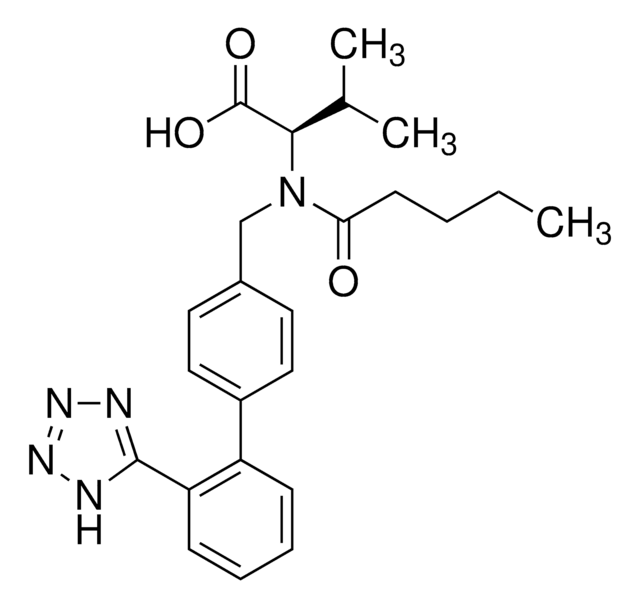
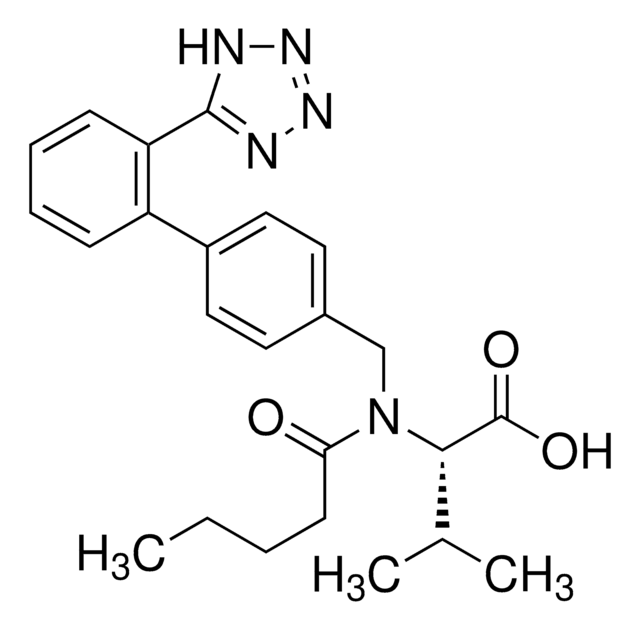
SMILES 字串
CCCC(=O)N(Cc1ccc(cc1)-c2ccccc2-c3nnn[nH]3)[C@@H](C(C)C)C(O)=O
InChI
1S/C23H27N5O3/c1-4-7-20(29)28(21(15(2)3)23(30)31)14-16-10-12-17(13-11-16)18-8-5-6-9-19(18)22-24-26-27-25-22/h5-6,8-13,15,21H,4,7,14H2,1-3H3,(H,30,31)(H,24,25,26,27)/t21-/m0/s1
InChI 密鑰
OKAQHVJSXLGXET-NRFANRHFSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the potent, highly selective, and orally active antagonist of the angiotensin II AT1-receptor, valsartan, used widely for the treatment of hypertension.
應用
- Development of a reversed-phase high-performance liquid chromatographic (RP-HPLC) method for the determination of valsartan and its related impurities in pharmaceutical dosage forms
- Impurity testing of valsartan, amlodipine besylate, and hydrochlorothiazide in their combined dosage form by a stability-indicating ultra-high performance liquid chromatography (UHPLC)
- Simultaneous determination of amlodipine and valsartan in their combined dosage form, in the presence of their degradation products by a gradient reversed phase-liquid chromatographic (RP-LC) method
- Separation and detection of nitrosamines and other related impurities in valsartan and losartan using supercritical fluid chromatography (SFC) in a single run
- Development and validation of a UHPLC method for the estimation of sacubitril, valsartan, and their related impurities in their combined dosage form, following ICH Q2 (R1) guideline
分析報告
腳註
訊號詞
Warning
危險聲明
危險分類
Repr. 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務