推薦產品
等級
pharmaceutical primary standard
API 家族
valsartan
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
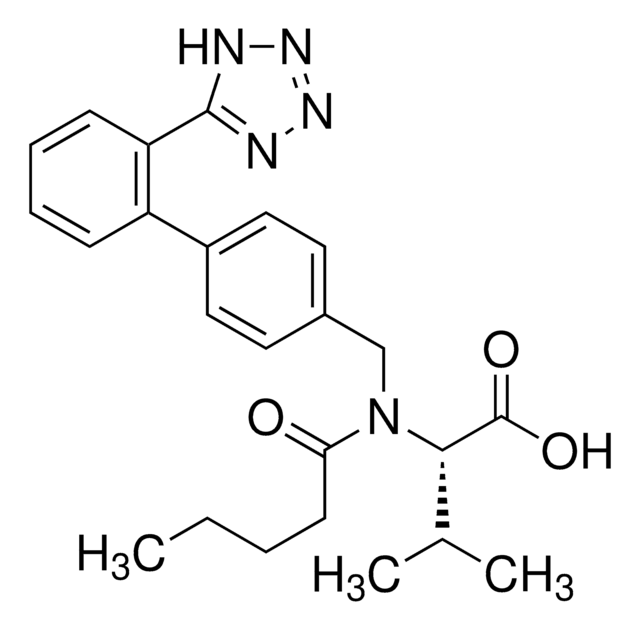
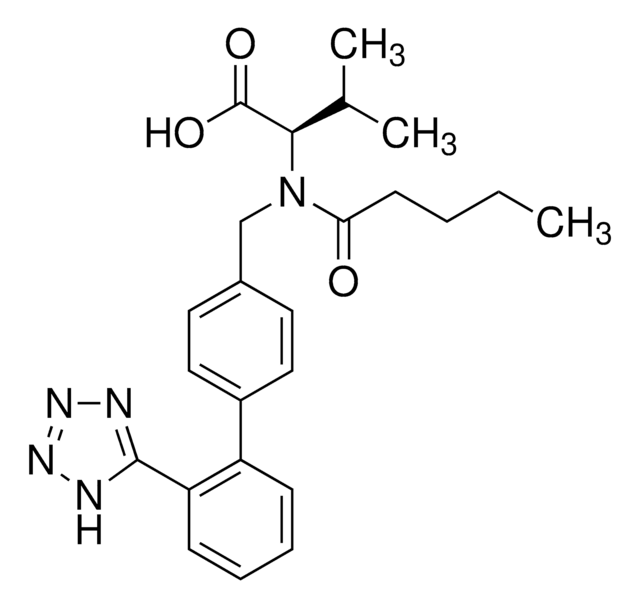
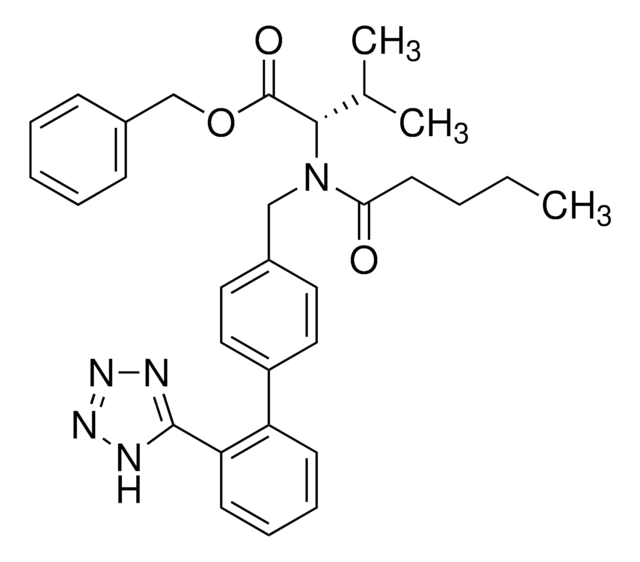
CCCCC(=O)N(Cc1ccc(cc1)-c2ccccc2-c3nnn[nH]3)[C@@H](C(C)C)C(O)=O
InChI
1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1
InChI 密鑰
ACWBQPMHZXGDFX-QFIPXVFZSA-N
基因資訊
human ... AGTR1(185)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Valsartan EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Repr. 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Qiuping Ma et al.
International journal of pharmaceutics, 441(1-2), 75-81 (2012-12-26)
The central purpose of this study was to evaluate the impact of drug particle size and crystalline state on valsartan (VAL) formulations in order to improve its dissolution and bioavailability. VAL microsuspension (mean size 22 μm) and nanosuspension (30-80nm) were
Abdul Ahad et al.
International journal of pharmaceutics, 443(1-2), 26-38 (2013-01-15)
The aim of the current investigation is to develop and statistically optimize nanoethosomes for transdermal valsartan delivery. Box-Behnken experimental design was applied for optimization of nanoethosomes. The Independent variables were phospholipids 90G (X(1)), ethanol (X(2)), valsartan (X(3)) and sonication time
Teun van der Bom et al.
Circulation, 127(3), 322-330 (2012-12-19)
The role of angiotensin II receptor blockers in patients with a systemic right ventricle has not been elucidated. We conducted a multicenter, double-blind, parallel, randomized controlled trial of angiotensin II receptor blocker valsartan 160 mg twice daily compared with placebo
Moko Zeniya et al.
Hypertension (Dallas, Tex. : 1979), 62(5), 872-878 (2013-09-11)
Na-K-Cl cotransporter isoform 1 (NKCC1) is involved in the regulation of vascular smooth muscle cell contraction. Recently, the with-no-lysine kinase (WNK)-STE20/SPS1-related proline/alanine-rich kinase (SPAK)-NKCC1 phosphorylation cascade in vascular smooth muscle cells was found to be important in the regulation of
Kwang Kon Koh et al.
Diabetes, 62(10), 3547-3552 (2013-07-19)
Statin and angiotensin II type 1 receptor blocker therapy improves endothelial dysfunction using distinct mechanisms. We evaluated simultaneous vascular and metabolic responses to pravastatin and valsartan therapy, alone or in combination, in hypercholesterolemic patients. Forty-eight hypercholesterolemic patients (23 had metabolic
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務