推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. Y0000049
traceable to USP 1029501
API 家族
amlodipine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
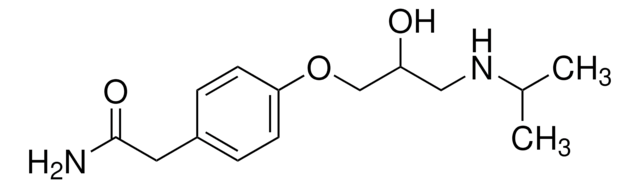
OS(=O)(=O)c1ccccc1.CCOC(=O)C2=C(COCCN)NC(C)=C(C2c3ccccc3Cl)C(=O)OC
InChI
1S/C20H25ClN2O5.C6H6O3S/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21;7-10(8,9)6-4-2-1-3-5-6/h5-8,17,23H,4,9-11,22H2,1-3H3;1-5H,(H,7,8,9)
InChI 密鑰
ZPBWCRDSRKPIDG-UHFFFAOYSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
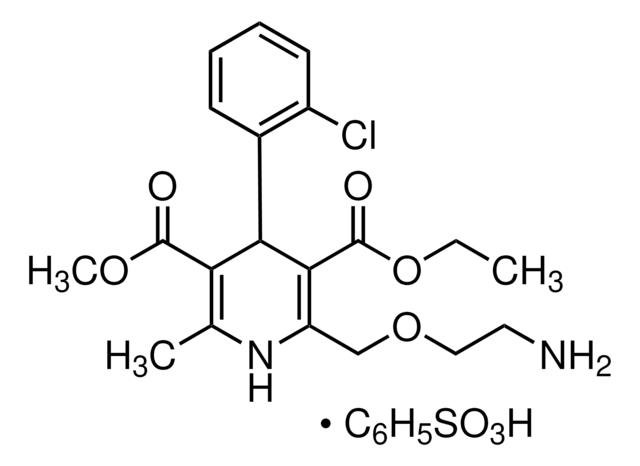
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Amlodipine besylate is a calcium channel blocker, which inhibits the trans membrane influx of calcium ions into vascular smooth muscles and cardiac muscle. It belongs to the dihydropyridine family. It is used in combination with atorvastatin calcium to treat vasospastic angina, chronic stable angina, hypertension, in elevated serum triglyceride levels, primary dysbetalipoproteinemia.
應用
Amlodipine besylate may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using different chromatography techniques and spectrophotometric technique.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
氨氯地平是一种 L 型钙通道阻滞剂。氨氯地平属于一类心血管药物,作用于 Ca V 1 或 L 型的电压门控钙通道。氨氯地平还具有降压和抗心绞痛作用。其活性主要存在于 (-)-异构体中。氨氯地平抑制人表皮样癌细胞 A431 的生长,对雄性大鼠有抗生殖作用。
苯磺酸氨氯地平是一种 L 型钙通道阻滞剂。
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC0717 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
客戶也查看了
Spectrophotometric and high performance liquid chromatographic determination of amlodipine besylate in pharmaceuticals
Basavaiah K, et al.
ScienceAsia, 31(1), 13-21 (2005)
Stability indicating RP-HPLC estimation of atorvastatin calcium and amlodipine besylate in pharmaceutical formulations
Shah AD, et al.
Indian Journal of Pharmaceutical Sciences, 70(6), 754-754 (2008)
Juliano Lara Fernandes et al.
The American journal of medicine, 126(9), 834-837 (2013-07-09)
Iron chelation therapy in patients with thalassemia major may not prevent iron overload in all organs, especially those in which iron enters cells through specific calcium channels. We designed a controlled pilot study to assess the potential of the calcium
Giuseppe Derosa et al.
Journal of the American Society of Hypertension : JASH, 7(1), 32-39 (2013-01-17)
The purpose of this study was to evaluate a fixed olmesartan/amlodipine combination on blood pressure control, lipid profile, insulin sensitivity, and some inflammatory markers compared with single-drug monotherapy. A total of 276 hypertensive patients were randomly assigned to olmesartan 20
László Bajnok
Orvosi hetilap, 154(7), 243-247 (2013-02-12)
From the evaluated ONTARGET, ALTITUDE, ACCOMPLISH, ROADMAP, and ACCORD-BP studies a conclusion can be drawn that though microalbuminuria/proteinuria is a strong epidemiological biomarker, in interventional studies it is not necessarily a reliable surrogate endpoint as actual renal function may change
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務