推薦產品
形狀
powder
包裝
pkg of 1 × 10 mg (860516P-10mg)
pkg of 1 × 100 mg (860516P-100mg)
pkg of 1 × 25 mg (860516P-25mg)
pkg of 1 × 5 mg (860516P-5mg)
pkg of 1 × 50 mg (860516P-50mg)
製造商/商標名
Avanti Research™ - A Croda Brand
脂質類型
sphingolipids
運輸包裝
dry ice
儲存溫度
−20°C
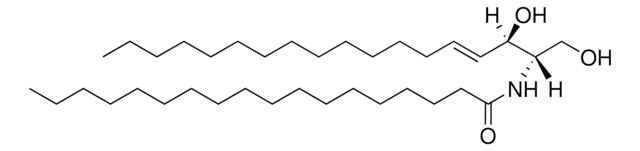
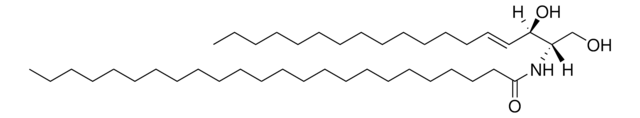
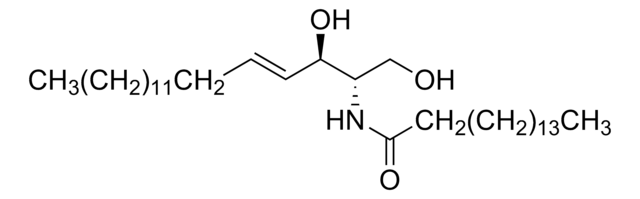
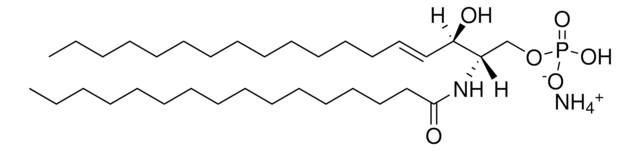
SMILES 字串
OC[C@]([H])(NC(CCCCCCCCCCCCCCC)=O)[C@]([H])(O)/C=C/CCCCCCCCCCCCC
InChI
1S/C34H67NO3/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-33(37)32(31-36)35-34(38)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h27,29,32-33,36-37H,3-26,28,30-31H2,1-2H3,(H,35,38)/b29-27+/t32-,33+/m0/s1
InChI 密鑰
YDNKGFDKKRUKPY-TURZORIXSA-N
一般說明
神经酰胺包含鞘氨醇和脂肪酸,属于鞘脂家族。
應用
C16神经酰胺(d18:1/16:0)已用于:
- 色谱及串联质谱负离子电喷雾电离方法联用定量测定心肌神经酰胺的内标物
- 作为液相色谱-质谱(MS/MS)联用法定量测定大鼠样品中神经酰胺的内标物
- 测试其对蠕虫线粒体应激反应的影响
生化/生理作用
在慢性酒精相关性肝病(ALD)中,C16神经酰胺水平升高。通常,神经酰胺抑制线粒体功能和胰岛素信号传导。它们是细胞膜的重要组成部分,并参与脂筏(lipid raft )的形成。C16神经酰胺的类似物(C2-C16 CCPS)是化疗中的潜在抗癌药。C16神经酰胺的水平及其与其它神经酰胺的比率被认为是预测冠状动脉疾病(CAD)的生物标志物。它与肿瘤抑制蛋白p53的DNA结合结构域相互作用,并激活p53。
包裝
5 mL琥珀色玻璃螺旋盖小瓶(860516P-100mg)
5 mL琥珀色玻璃螺旋盖小瓶(860516P-10mg)
5 mL琥珀色玻璃螺旋盖小瓶(860516P-25mg)
5 mL琥珀色玻璃螺旋盖小瓶(860516P-50mg)
5 mL琥珀色玻璃螺旋盖小瓶(860516P-5mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
也與該產品經常一起購買
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
C 16-ceramide is a natural regulatory ligand of p53 in cellular stress response
Fekry B, et al.
Nature Communications, 9(1), 4149-4149 (2018)
Aritz B García-Arribas et al.
Langmuir : the ACS journal of surfaces and colloids, 32(35), 9053-9063 (2016-08-04)
The effects of increasing amounts of palmitoylceramide (pCer) on human red blood cell lipid membranes have been studied using atomic force microscopy of supported lipid bilayers, in both imaging (bilayer thickness) and force-spectroscopy (nanomechanical resistance) modes. Membranes appeared homogeneous with
M Laura Fanani et al.
Langmuir : the ACS journal of surfaces and colloids, 34(39), 11749-11758 (2018-09-06)
Sphingosine [(2 S,3 R,4 E)-2-amino-4-octadecene-1,3-diol] is the most common sphingoid base in mammals. Ceramides are N-acyl sphingosines. Numerous small variations on this canonical structure are known, including the 1-deoxy, the 4,5-dihydro, and many others. However, whenever there is a Δ4
C16-ceramide analog combined with Pc 4 photodynamic therapy evokes enhanced total ceramide accumulation, promotion of DEVDase activation in the absence of apoptosis, and augmented overall cell killing
Separovic D, et al.
Journal of Lipids, 2011 (2011)
Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease
Longato L, et al.
Oxidative Medicine and Cellular Longevity, 2012 (2012)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務