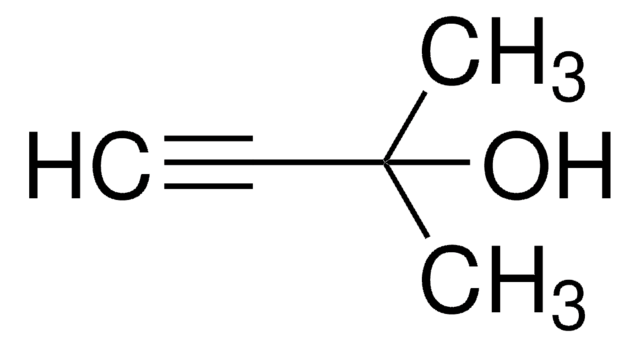
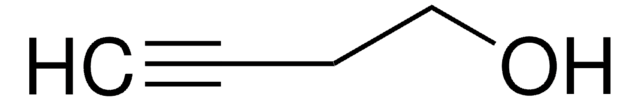
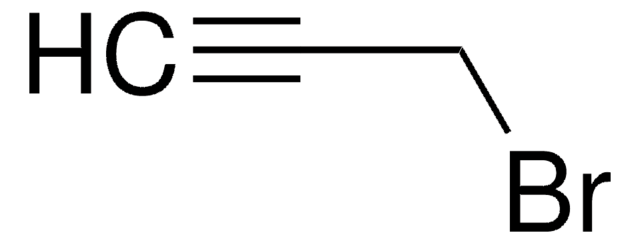
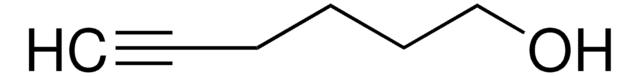
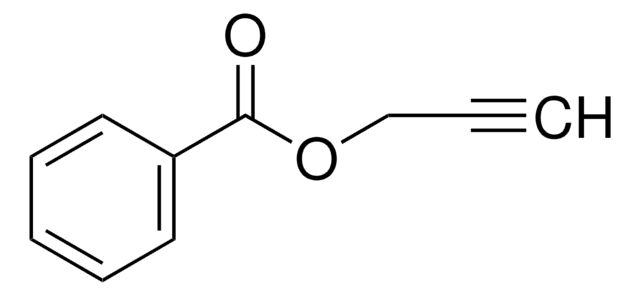
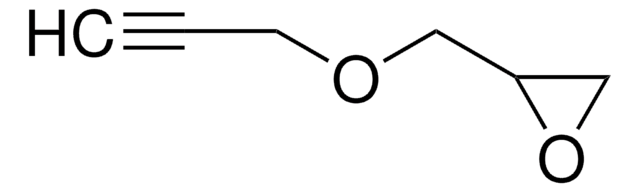
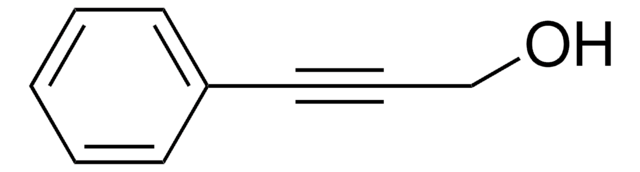推薦產品
蒸汽密度
1.93 (vs air)
品質等級
蒸汽壓力
11.6 mmHg ( 20 °C)
化驗
99%
折射率
n20/D 1.432 (lit.)
bp
114-115 °C (lit.)
mp
−53 °C (lit.)
密度
0.963 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
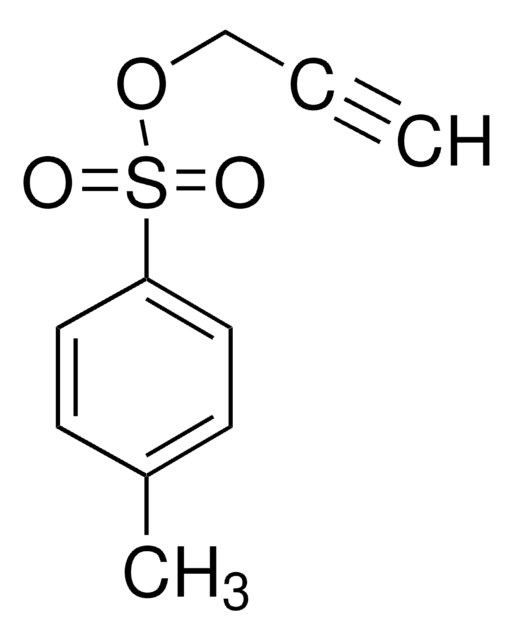
SMILES 字串
OCC#C
InChI
1S/C3H4O/c1-2-3-4/h1,4H,3H2
InChI 密鑰
TVDSBUOJIPERQY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Propargyl alcohol has been used as a key starting material in the [4+2] cycloisomerization mediated synthesis of various phthalide derivatives.
It can also be used to synthesize:
It can also be used to synthesize:
- A variety of regioselective furan-3-carboxamides by reacting with 3-oxo amides using Ag2CO3 as a promoter.
- β-oxopropyl esters by reacting with carboxylic acids in the presence of (arene) (phosphine)ruthenium(II) complex as a catalyst.
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT RE 2
標靶器官
Liver,Kidney
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
91.4 °F - closed cup
閃點(°C)
33 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Ming Chen et al.
Journal of the American Chemical Society, 134(26), 10947-10952 (2012-06-27)
Chiral Brønsted acid catalyzed asymmetric allenylboration reactions are described. Under optimized conditions, anti-homopropargyl alcohols 2 are obtained in high yields with excellent diastereo- and enantioselectivities from stereochemically matched aldehyde allenylboration reactions with (M)-1 catalyzed by the chiral phosphoric acid (S)-4.
Lu Wang et al.
Organic letters, 14(23), 5848-5851 (2012-11-14)
A straightforward synthesis of fully substituted β-carbolines via Brønsted acid promoted cyclizations of α-indolyl propargylic alcohols with nitrones is described. The use of nitrones bearing alkenyl or electron-rich aryl groups as the R(4) substituent dramatically switches the reaction pathway to
Hua-Dong Xu et al.
Organic letters, 15(4), 840-843 (2013-01-30)
Cross-dimerization of terminal arylacetylenes and terminal propargylic alcohols/amides has been achieved in the presence of a rhodium catalyst. This method features high chemo- and regioselectivities rendering convenient and atom economical access to functionalized enynes.
Silver (i)/base-promoted propargyl alcohol-controlled regio-or stereoselective synthesis of furan-3-carboxamides and (Z)-enaminones.
Sultana S, et al.
Organic & Biomolecular Chemistry, 16(36), 6749-6759 (2018)
Srijit Biswas et al.
Chemical communications (Cambridge, England), 48(52), 6586-6588 (2012-05-25)
A one-step atom efficient gold(I)-catalyzed route to α-sulfenylated ketones and aldehydes from propargylic alcohols and aryl thiols is described.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務