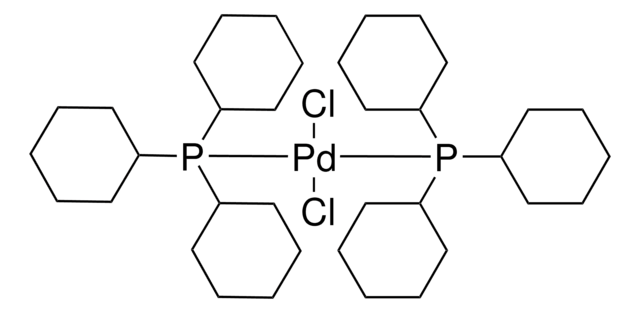
About This Item
推薦產品
品質等級
化驗
98%
形狀
solid
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
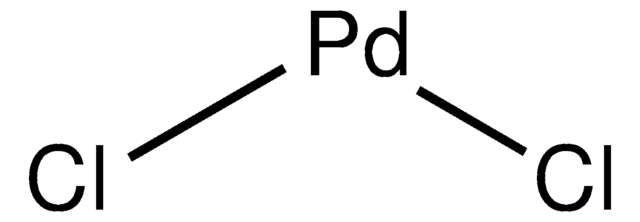
SMILES 字串
Cl[Pd]Cl.c1(P(c2ccccc2)c3ccccc3)ccccc1.c4(P(c5ccccc5)c6ccccc6)ccccc4
InChI
1S/2C18H15P.2ClH.Pd/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2
InChI 密鑰
YNHIGQDRGKUECZ-UHFFFAOYSA-L
尋找類似的產品? 前往 產品比較指南
一般說明
應用
- 2-碘苯甲醚和末端炔烃偶联合成 2,3-二取代苯并呋喃的反应。
- 用于合成二苯乙炔的无铜Sonogashira 交叉偶联反应
- 苯乙烯的区域选择性氢羧基化。
- 氟芳基特戊酸锌的Negishi偶联以制备氟化寡苯(fluorinated oligophenyl)。
- 烃的碘-α-β-未取代酯的偶联以生成四取代烯。
相關產品
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
文章
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務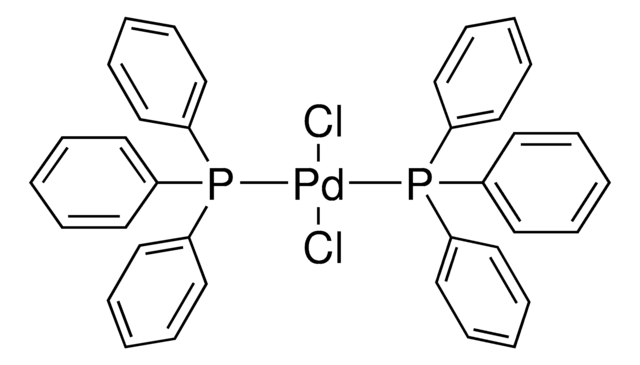


![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)