推薦產品
形狀
solid
品質等級
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
275-280 °C
SMILES 字串
[Fe].ClCCl.Cl[Pd]Cl.[CH]1[CH][CH][C]([CH]1)P(c2ccccc2)c3ccccc3.[CH]4[CH][CH][C]([CH]4)P(c5ccccc5)c6ccccc6
InChI
1S/2C17H14P.CH2Cl2.2ClH.Fe.Pd/c2*1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;2-1-3;;;;/h2*1-14H;1H2;2*1H;;/q;;;;;;+2/p-2
InChI 密鑰
CWHRHCATIKFSND-UHFFFAOYSA-L
尋找類似的產品? 前往 產品比較指南
分析報告
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 379670-1G | 4061831837346 |
| 379670-25G | 4061831935752 |
| 379670-5G | 4061831951301 |
| 379670-250G | 4061833398395 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-双(二-叔丁基膦基)二茂铁]二氯合钯(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)


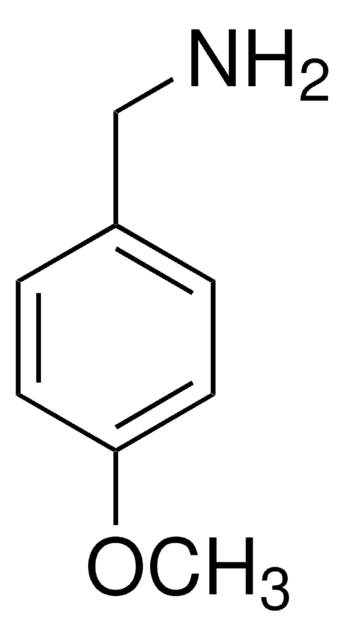


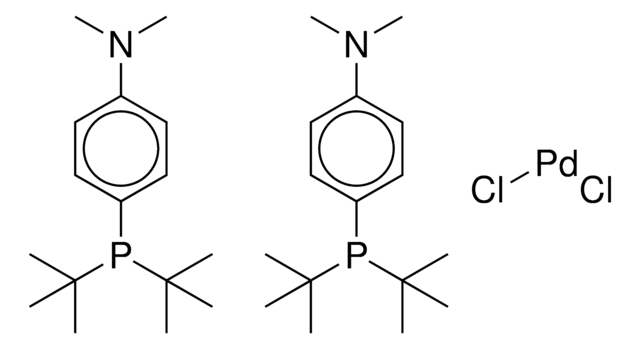

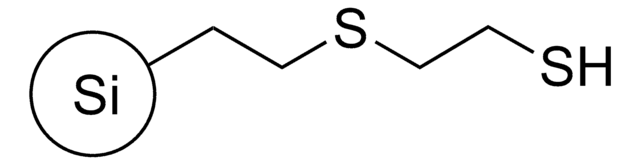

![[1,1′-双(二苯基膦)二茂铁]二氯化镍(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)