推薦產品
品質等級
化驗
98%
包含
≤2.5% water
折射率
n20/D 1.449 (lit.)
bp
83 °C (lit.)
密度
0.86 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
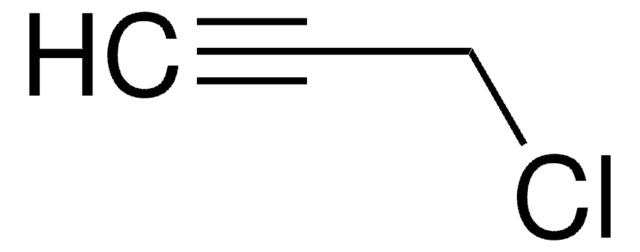
SMILES 字串
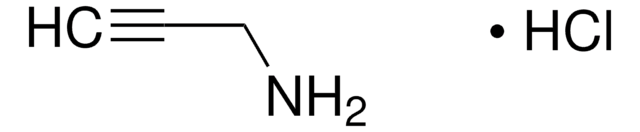
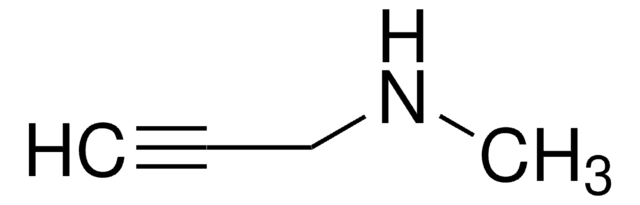
NCC#C
InChI
1S/C3H5N/c1-2-3-4/h1H,3-4H2
InChI 密鑰
JKANAVGODYYCQF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
通过碳水化合物连接型氨基酸的炔丙基酰胺与 9,10-双(叠氮甲基)蒽进行 1,3-偶极环加成反应,合成手性、荧光大环类化合物。
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
42.8 °F - closed cup
閃點(°C)
6 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Synthesis, 3141-3141 (2006)
Simona Pilotto et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(9), 2752-2757 (2015-03-03)
With its noncatalytic domains, DNA-binding regions, and a catalytic core targeting the histone tails, LSD1-CoREST (lysine-specific demethylase 1; REST corepressor) is an ideal model system to study the interplay between DNA binding and histone modification in nucleosome recognition. To this
Irene Bolea et al.
Journal of neural transmission (Vienna, Austria : 1996), 120(6), 893-902 (2012-12-15)
Alzheimer's disease (AD) is a complex neurodegenerative disorder with a multifaceted pathogenesis. There are at present three Food and Drug Administration-approved drugs based on the "one drug, one target" paradigm (donepezil, galantamine and rivastigmine) that improve symptoms by inhibiting acetylcholinesterase.
Olga P Pereshivko et al.
Organic letters, 12(11), 2638-2641 (2010-05-06)
An efficient, microwave-assisted Cu(I)-catalyzed one-pot coupling of a ketone, an alkyne, and a primary amine (KA(2) coupling) is described, giving access to secondary propargylamines.
Parminder Kaur et al.
Organic & biomolecular chemistry, 8(5), 1091-1096 (2010-02-19)
A variety of substituted chiral propargylamines have been synthesized by reacting chiral N-phosphonylimines with lithium aryl/alkyl acetylides. Seventeen examples were studied to give excellent yields (>90%) and diastereoselectivities (96 : 4 to 99 : 1). It was found that the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務