推薦產品
品質等級
化驗
97% (HPLC)
形狀
solid
mp
44-49 °C
官能基
fluoro
sulfone
SMILES 字串
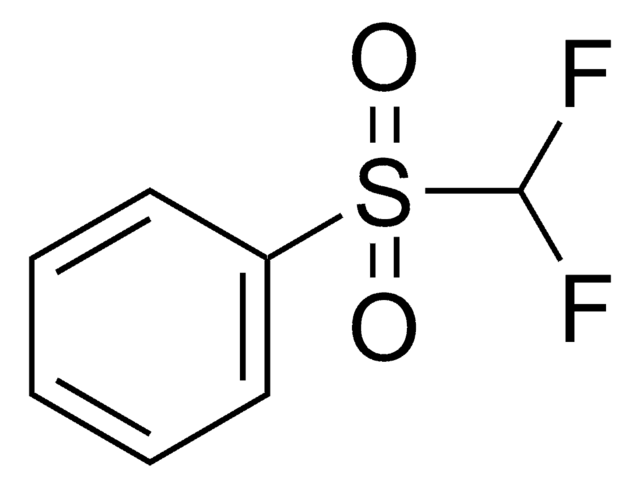
O=S(C1=CC=CC=N1)(C(F)F)=O
InChI
1S/C6H5F2NO2S/c7-6(8)12(10,11)5-3-1-2-4-9-5/h1-4,6H
InChI 密鑰
YRQNSTAWTLXCEZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Difluoromethyl 2-pyridyl sulfone (2-PySO2CF2H) is a reagent used in the gem-difluoroolefination of aldehydes and ketones. It is also used as a reagent in the nucleophilic difluoro(sulfonato)methylation of alcohols, N-sulfinyl imines, and halides.
應用
Reagent is used in the olefination of ketones and aldehydes to form gem-difluoro olefins under basic conditions. Product is also used as a crucial intermediate toward making 1,1-difluorinated alkyl chains for the alkylation of heterocycles.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Difluoromethyl 2-pyridyl sulfone: a versatile carbonyl gem-difluoroolefination reagent
Gao B, et al.
Organic Chemistry Frontiers : An International Journal of Organic Chemistry / Royal Society of Chemistry, 2(2), 163-168 (2015)
From difluoromethyl 2-pyridyl sulfone to difluorinated sulfonates: a protocol for nucleophilic difluoro(sulfonato)methylation.
G K Surya Prakash et al.
Angewandte Chemie (International ed. in English), 50(11), 2559-2563 (2011-03-04)
Yanchuan Zhao et al.
Organic letters, 12(7), 1444-1447 (2010-03-10)
Difluoromethyl 2-pyridyl sulfone, a previously unknown compound, was found to act as a novel and efficient gem-difluoroolefination reagent for both aldehydes and ketones. It was found that the fluorinated sulfinate intermediate in the reaction is relatively stable, which can be
Direct synthesis of fluorinated heteroarylether bioisosteres.
Qianghui Zhou et al.
Angewandte Chemie (International ed. in English), 52(14), 3949-3952 (2013-03-06)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務