推薦產品
化驗
≥97%
形狀
solid
官能基
fluoro
sulfone
SMILES 字串
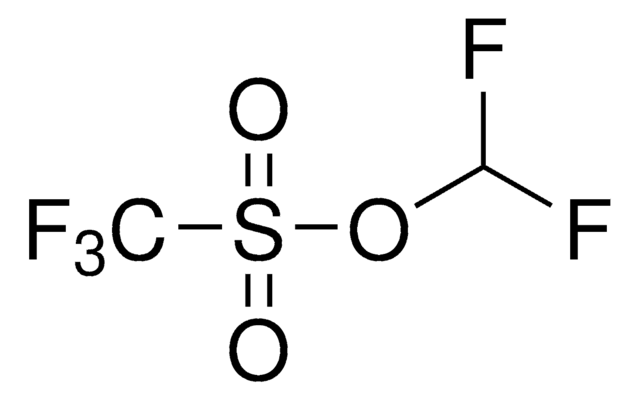
FC(F)S(=O)(=O)c1ccccc1
InChI
1S/C7H6F2O2S/c8-7(9)12(10,11)6-4-2-1-3-5-6/h1-5,7H
InChI 密鑰
LRHDNAVPELLXDL-UHFFFAOYSA-N
應用
Reagent Used for
Reagent used in Preparation of
- Reductive silylation and the preparation of trifluoro- and difluoromethylsilanes by reductive coupling of fluoromethyl sulfones, sulfoxides and sulfides with chlorosilanes
- Fluoroalkylation/chloroalkylation of α,β-enones, arynes, acetylenic ketones and other Michael acceptors
- Difluoromethylation of primary alkyl halides via nucleophilic substitution-reductive desulfonylation
Reagent used in Preparation of
- α-difluoromethyl amines via stereoselective (phenylsulfonyl)difluoromethylation of chiral sulfinyl aldimines
- Anti-difluoropropanediols via potassium tert-butoxide-catalyzed difluoromethylenation of aldehydes
- β-difluoromethylated and β-difluoromethylenated alcohols and amines by regioselective nucleophilic difluoromethylation of 1,2-cyclic sulfates and sulfamidates
- Difluoroalkenes from alkyl halides via nucleophilic substitution-elimination
- Difluoromethyl alcohol derivatives from enolizable and non-enolizable carbonyl compounds using nucleophilic phenylsulfonyldifluoromethylation-reductive desulfonylation strategy
- Fluoromethylated vicinal ethylenediamines via fluoromethylation of chiral α-aminobutanesulfinimines with (phenylsulfonyl)fluoromethanes followed by reductive desulfonylation and alcoholysis
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
264.0 °F
閃點(°C)
128.9 °C
Jun Liu et al.
The Journal of organic chemistry, 72(8), 3119-3121 (2007-03-28)
The diastereoselective nucleophilic (phenylsulfonyl)difluoromethylation and (phenylsulfonyl)monofluoromethylation of alpha-amino N-tert-butanesulfinimines (3) by using PhSO2CF2H and PhSO2CH2F reagents gave products 4 or 5 in high yields (73-99%) and with excellent diastereoselectivity (dr up to >99:1). After subsequent reductive desulfonylation and acid-catalyzed alcoholysis
A remarkably efficient fluoroalkylation of cyclic sulfates and sulfamidates with PhSO2CF2H: facile entry into beta-difluoromethylated or beta-difluoromethylenated alcohols and amines.
Chuanfa Ni et al.
Angewandte Chemie (International ed. in English), 46(5), 786-789 (2006-12-14)
Difluoromethyl phenyl sulfone as a selective difluoromethylene dianion equivalent: one-pot stereoselective synthesis of anti-2,2-difluoropropane-1,3-diols.
G K Surya Prakash et al.
Angewandte Chemie (International ed. in English), 42(42), 5216-5219 (2003-11-06)
Convenient synthesis of difluoromethyl alcohols from both enolizable and non-enolizable carbonyl compounds with difluoromethyl phenyl sulfone
Prakash, G. K. S.; et al.
European Journal of Organic Chemistry, 11, 2218-2223 (2005)
Difluoromethyl phenyl sulfone, a difluoromethylidene equivalent: use in the synthesis of 1,1-difluoro-1-alkenes.
G K Surya Prakash et al.
Angewandte Chemie (International ed. in English), 43(39), 5203-5206 (2004-09-30)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務