全部照片(1)
About This Item
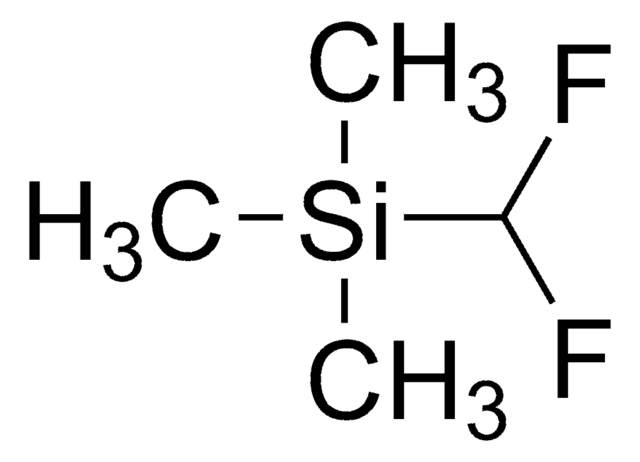
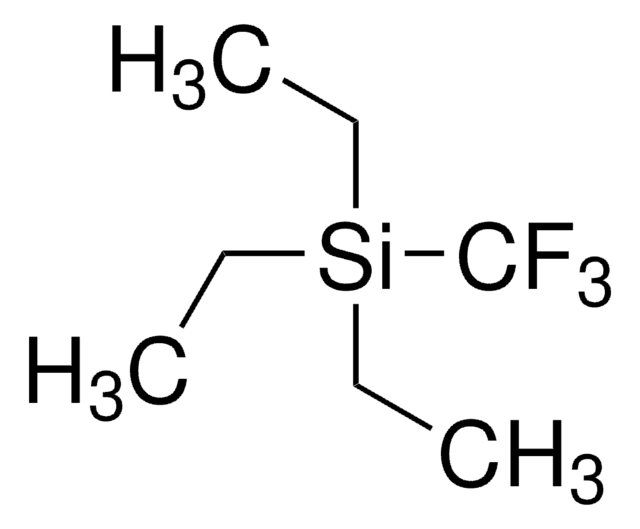
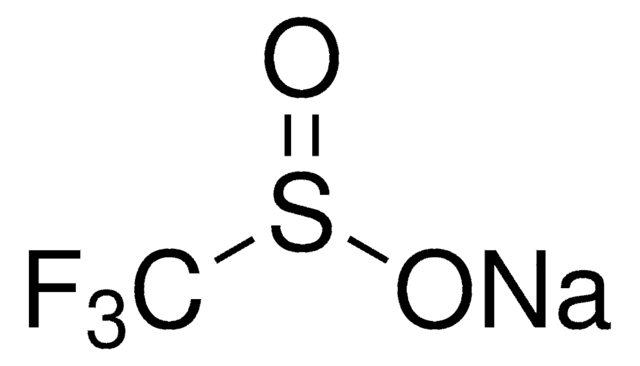
線性公式:
(CH3)3SiCF3
CAS號碼:
分子量::
142.19
Beilstein:
4241868
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推薦產品
應用
三甲基(三氟甲基)硅烷可以在以下过程中用作三氟甲基化剂:
- 将 N-(叔-丁基亚磺酰基)-亚胺转化为三氟甲基化胺
- 将反式-烯酮转化为反式-α-三氟甲基甲硅烷基醚
- 偶氮甲亚胺的三氟甲基化
- 将H-膦酸酯转化为CF3-膦酸酯
- 三氟甲基通过亲核加成转化成醛和酮。
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2 - Water-react 2
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves
客戶也查看了
Craig P Johnston et al.
Journal of the American Chemical Society, 140(35), 11112-11124 (2018-08-07)
The mechanism of CF3 transfer from R3SiCF3 (R = Me, Et, iPr) to ketones and aldehydes, initiated by M+X- (<0.004 to 10 mol %), has been investigated by analysis of kinetics (variable-ratio stopped-flow NMR and IR), 13C/2H KIEs, LFER, addition
Catalytic enantioselective trifluoromethylation of azomethine imines with trimethyl (trifluoromethyl) silane
Kawai H, et al.
Angewandte Chemie (International Edition in English), 121(34), 6442-6445 (2009)
Peter T Kaplan et al.
Beilstein journal of organic chemistry, 13, 2297-2303 (2017-11-29)
A number of copper reagents were compared for their effectiveness in trifluoromethylating 4-iodobiphenyl, 4-iodotoluene, and 2-iodotoluene. Yields over time were plotted in order to refine our understanding of each reagent performance, identify any bottlenecks, and provide more insight into the
Stereoselective Nucleophilic Trifluoromethylation of N?(tert?Butylsulfinyl) imines by Using Trimethyl (trifluoromethyl) silane.
Prakash G K, et al.
Angewandte Chemie (International Edition in English), 113(3), 609-610 (2001)
CsF-catalyzed nucleophilic trifluoromethylation of trans-enones with trimethyl (trifluoromethyl) silane: A facile synthesis of trans-?-trifluoromethyl allylic alcohols.
Singh R P, et al.
Organic Letters, 1(7), 1047-1049 (1999)
文章
Reagents for C–C Bond Formation
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務