推薦產品
等級
reagent grade
品質等級
蒸汽壓力
1 mmHg ( 220 °C)
化驗
≥98%
形狀
solid
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
256-258 °C (dec.) (lit.)
環保替代類別
SMILES 字串
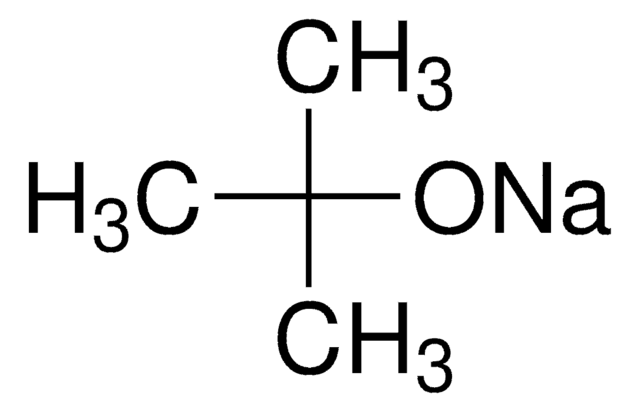
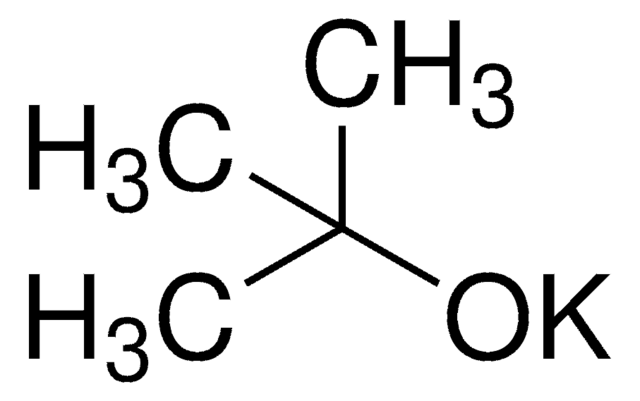
[K+].CC(C)(C)[O-]
InChI
1S/C4H9O.K/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI 密鑰
LPNYRYFBWFDTMA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
我们致力于为您提供更环保的替代产品,以符合“绿色化学的12项原则”的一项或多项原则要求。该产品为增强型,提高了催化效率。点击此处,查看更多详情。
應用
叔丁醇钾已作为强碱,用于通过N-叔丁基亚磺酰亚胺的转移氢化反应合成高对映体选择性的胺。
它也可用于:
它也可用于:
- 由酯和胺合成相应的脂肪和芳香酰胺。
- 在芳基醚、胺和酰胺的分子内环化中用作碱。
- 作为催化剂,通过芳基卤和烯烃的Mizoroki-Heck反应制备苯乙烯衍生物。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 2 - Skin Corr. 1A
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Mizoroki-Heck-type reaction mediated by potassium tert-butoxide
Shirakawa E, et al.
Angewandte Chemie (International ed. in English), 123(20), 4767-4770 (2011)
"Potassium tert-Butoxide Promoted Intramolecular Arylation via a Radical Pathway"
Roman SD, et al.
Organic Letters, 13(12), 3242-3245 (2011)
tert-Butoxide-assisted amidation of esters under green conditions
Kim Bo Ram, et al.
Synthesis, 44(01), 42-50 (2012)
Hajime Ito et al.
Chemical communications (Cambridge, England), 48(64), 8006-8008 (2012-07-10)
The regio- and diastereoselective silaboration of aromatic alkenes with a silylboron compound proceeds in the presence of a catalytic amount of potassium tert-butoxide, providing a complementary method to the corresponding transition metal-catalyzed reactions.
Aurélie Mallinger et al.
The Journal of organic chemistry, 74(3), 1124-1129 (2008-12-24)
3-Aryltetronic acids were prepared in one step by treatment of a mixture of methyl arylacetates and methyl hydroxyacetates with potassium tert-butoxide, via tandem transesterification/Dieckmann condensation. Several mushroom or lichen pigments, vulpinic acids, were synthesized from 3-(4-methoxyphenyl)tetronic acid in three steps
條款
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 156671-25G | 4061838742858 |
| 156671-5G | 4061833410028 |
| 156671-100G | 4061838742834 |
| 156671-2.5KG | 4061838742841 |
| 156671-500G | 4061838742865 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務