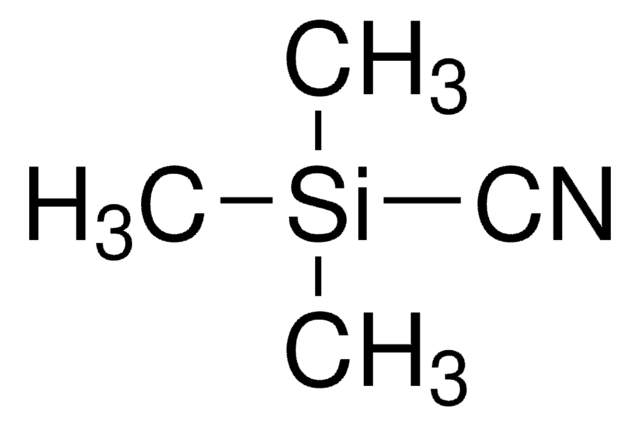
推薦產品
等級
technical
品質等級
化驗
≥95% (GC)
形狀
liquid
折射率
n20/D 1.392 (lit.)
bp
114-117 °C (lit.)
mp
8-11 °C (lit.)
密度
0.793 g/mL at 20 °C (lit.)
SMILES 字串
C[Si](C)(C)C#N
InChI
1S/C4H9NSi/c1-6(2,3)4-5/h1-3H3
InChI 密鑰
LEIMLDGFXIOXMT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Trimethylsilyl cyanide (TMSCN) can be used as a reagent in the:
- Cyanosilylation of carbonyl compounds using various catalysts.
- Synthesis of α-aminonitriles by one-pot, three-component Strecker reaction of ketones with various amines using Brønsted acid catalyst.
- Cyanation of aryl halides using palladium-complex as a catalyst.
訊號詞
Danger
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
33.8 °F - closed cup
閃點(°C)
1 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Indium tribromide: a highly effective catalyst for the addition of trimethylsilyl cyanide to ?-hetero-substituted ketones
Bandini M, et al.
Tetrahedron Letters, 42(16), 3041-3043 (2001)
Asymmetric addition of trimethylsilyl cyanide to ketones catalyzed by Al (salen)/triphenylphosphine oxide
Kim SS and Kwak Ju M
Tetrahedron, 62(1), 49-53 (2006)
A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide
Sundermeier M, et al.
Journal of Organometallic Chemistry, 684(1-2), 50-55 (2003)
Mikhail D Kosobokov et al.
The Journal of organic chemistry, 77(13), 5850-5855 (2012-06-20)
A new silicon reagent, difluoro(trimethylsilyl)acetonitrile, was prepared by insertion of difluorocarbene into silyl cyanide. The obtained silane served as a good cyanodifluoromethylating reagent toward aldehydes, N-tosylimines, N-alkylimines, and enamines under basic or acidic conditions.
Enantioselective synthesis of tertiary α-hydroxy phosphonates catalyzed by carbohydrate/cinchona alkaloid thiourea organocatalysts.
Shasha Kong et al.
Angewandte Chemie (International ed. in English), 51(35), 8864-8867 (2012-08-01)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務