推薦產品
一般說明
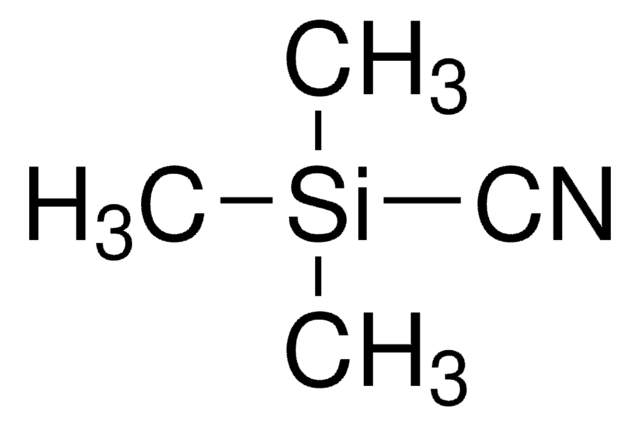
三甲基氰硅烷通过α-甲硅烷氧基腈参与羰基氨甲基化。
應用
三甲基氰硅烷被用于:
- 通过GC-MS对复杂代谢物混合物进行衍生化
- 作为脂肪族N,N-二烷基腙的对映选择性有机催化Strecker型反应的氰化物源
- 在亚甲硅基桥联稀土氧化物配合物催化的近定量产量醛的氰基甲硅烷基化反应中作为试剂
- 与手性双核Ti(IV)配合物的不对称氰基甲硅烷基化反应
包裝
包装材料含有颗粒状碳酸钠干燥剂。
訊號詞
Danger
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
33.8 °F - closed cup
閃點(°C)
1 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Bekzod Khakimov et al.
Analytical and bioanalytical chemistry, 405(28), 9193-9205 (2013-10-05)
Reproducible and quantitative gas chromatography-mass spectrometry (GC-MS)-based metabolomics analysis of complex biological mixtures requires robust and broad-spectrum derivatization. We have evaluated derivatization of complex metabolite mixtures using trimethylsilyl cyanide (TMSCN) and the most commonly used silylation reagent N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA). For
Aurora Martínez-Muñoz et al.
Organic & biomolecular chemistry, 11(47), 8247-8255 (2013-10-30)
The enantioselective organocatalytic Strecker-type reaction of aliphatic N,N-dialkylhydrazones is presented. Using trimethylsilyl cyanide (TMSCN) as the cyanide source, the reaction can be efficiently catalyzed by a tert-leucine-derived bifunctional thiourea to afford the corresponding hydrazino nitriles in good to excellent yields
Synthetic applications of trimethylsilyl cyanide. Efficient synthesis of. beta.-aminomethyl alcohols.
Evans DA, et al.
The Journal of Organic Chemistry, 39(7), 914-917 (1974)
Tetrahedron Asymmetry, 17, 2328-2328 (2006)
Synthetic Communications, 36, 2483-2483 (2006)
文章
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Reagents for C–C Bond Formation
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務