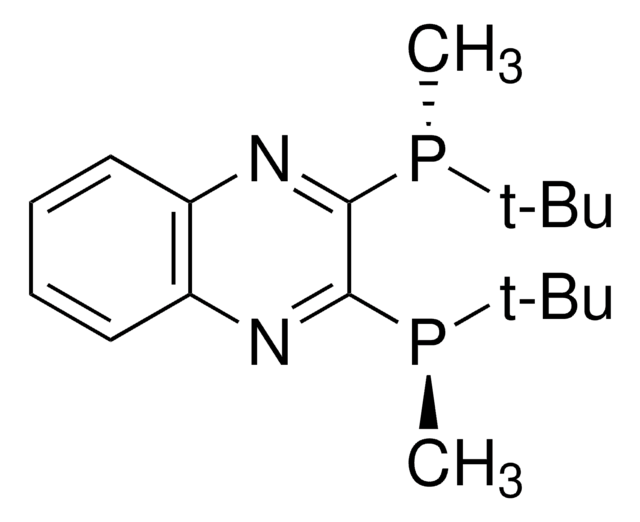
About This Item
推薦產品
品質等級
化驗
96%
形狀
solid
光學活性
[α]/D -34±4°, c = 1 in chloroform
mp
222-226 °C
官能基
phosphine
SMILES 字串
P(c7ccccc7)(c6ccccc6)c1c2ccc(c1)CCc3c(cc(cc3)CC2)P(c5ccccc5)c4ccccc4
InChI
1S/C40H34P2/c1-5-13-35(14-6-1)41(36-15-7-2-8-16-36)39-29-31-21-25-33(39)27-23-32-22-26-34(28-24-31)40(30-32)42(37-17-9-3-10-18-37)38-19-11-4-12-20-38/h1-22,25-26,29-30H,23-24,27-28H2
InChI 密鑰
GYZZZILPVUYAFJ-UHFFFAOYSA-N
應用
- Enantioselective reductive cyclization of 1,6-enynes via asymmetric hydrogenation in the presence of a rhodium catalyst to form alkylidene-substituted heterocycles.
- Asymmetric hydroboration of 3,3-disubstituted cyclopropenes to form 2,2-disubstituted cyclopropyl boronates.
- Asymmetric ring-opening reactions of azabenzonorbornadienes in the presence of zinc(II) triflate and palladium(II) acetate to form aminodihydronaphthalenes.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
文章
The P-Phos ligand family was developed by Professor Chan of Hong Kong Polytechnic University and licensed to JM CCT in 2002. P-Phos is an atropisomeric biaryl bisphosphine with the unique feature of incorporating two methoxy-substituted pyridine rings in the backbone.
We present an article concerning P-Phos, PhanePhos and BoPhoz™ Ligands.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![(S)-(+)-4,12-双(二苯基膦)-[2.2]-对环芳烷 96%](/deepweb/assets/sigmaaldrich/product/structures/396/009/d814b698-3227-4aef-b415-cb5f5730aa13/640/d814b698-3227-4aef-b415-cb5f5730aa13.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![1,1′-双[(2R,5R)-2,5-二乙基膦烷基]二茂铁](/deepweb/assets/sigmaaldrich/product/structures/163/400/0628df6f-e028-4669-8def-26e6eb4f4743/640/0628df6f-e028-4669-8def-26e6eb4f4743.png)
![[2.2]对环芳烷 97%](/deepweb/assets/sigmaaldrich/product/structures/165/940/d2dda3d5-1fe9-4c87-9a85-009490e67661/640/d2dda3d5-1fe9-4c87-9a85-009490e67661.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)